Figures & data
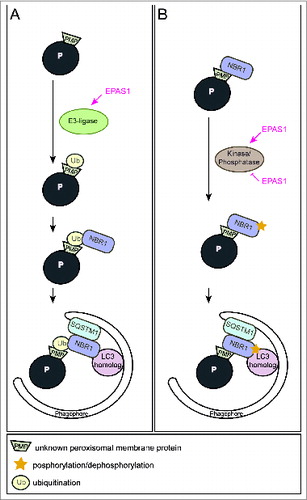
Figure 1. Two alternative models illustrating how EPAS1 might trigger pexophagy. (A) EPAS1 might induce an E3 ubiquitin ligase that specifically ubiquitinates a peroxisomal membrane protein that enhances the recruitment of the autophagy receptor NBR1 to the peroxisome surface. Accumulation of NBR1 on peroxisomes likely recruits SQSTM1, which was suggested to act as pexophagy co-receptor, and subsequently leads to clustering of peroxisomes via oligomerization of receptor-bound organelles. Accumulation of a critical mass of autophagy receptors at peroxisomes might concentrate sufficient ubiquitin-like modifiers (e.g., LC3 and GABARAPs) in close proximity to peroxisomes and prime phagophore assembly. (B) NBR1 could also be recruited to peroxisomes independently of ubiquitin via its membrane-interacting amphipathic α-helical J domain. In fact, NBR1 already localizes to peroxisomes in wild-type livers where pexophagy is not induced. EPAS1 might induce or inhibit a kinase/phosphatase that leads to a change in the posttranslational modification of peroxisome-bound NBR1 and thereby triggers pexophagy.