Figures & data
Figure 1. Valproic acid interacted synergistically with chemotherapeutic agents to inhibit lymphoma cell growth. (A and B) Cell growth determined by MTT assay (A) and Combination Index (CI) curve calculated by compusyn software (B) in T-lymphoma cell lines (Jurkat, H9) and B-lymphoma cell lines (SU-DHL-4, Nalmawa) treated with valproic acid (VPA) and/or doxorubicin (DOX) at 48 h. (C) Colony formation assay of Jurkat and SU-DHL-4 cells treated with VPA and DOX, either alone or in combination. Representative results shown in cells treated with VPA (0.5 mM) and/or DOX (15 nM) at 48 h. (D) CI curve of Jurkat and SU-DHL-4 cells treated with VPA and chemotherapeutic agents like cyclophosphamide, cisplatin, or gemcitabine at 48 h.
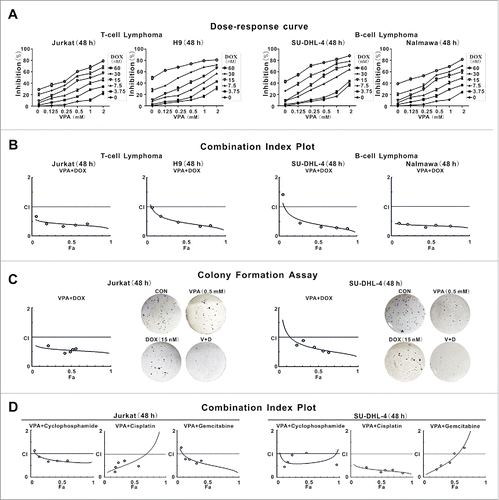
Figure 2. Valproic acid combined with doxorubicin induced lymphoma cell autophagy. (A) LC3B intensity determined by flow cytometry in T-lymphoma cell lines (Jurkat, H9) and B-lymphoma cell lines (SU-DHL-4, Nalmawa) treated with valproic acid (VPA, 0.5 mM) and/or doxorubicin (DOX, 15 nM) at 48 h. (B) The protein expression levels of LC3B-II detected by western blot in lymphoma cells treated with VPA (0.5 mM) and DOX (15 nM), either alone or in combination at 24 h (upper panels) and at 48 h (with BECN1 and SQSTM1 expression, lower panels). ACTB was used to assess equivalent protein loading. (C) Representative autophagic structures and quantitative data obtained by transmission electron microscopy in Jurkat and SU-DHL-4 cells treated with VPA (0.5 mM) and/or DOX (15 nM) at 48 h. Scale bars: 0.5 μm. **, P < 0.01, *, P < 0.05 compared with the CON (untreated) cells. (D) Growth inhibition determined by MTT assay in Jurkat cells treated with the autophagy inhibitor 3-methyladenine (0.5 mM, left panels) and BafA1 (10 nM, right panels). **, P < 0.01, *, P < 0.05 compared with the absence of inhibitor. (E) Growth inhibition determined by MTT assay in Jurkat cells treated with ATG5 siRNA (left panels) and BECN1 siRNA (right panels). **, P < 0.01, *, P < 0.05 compared with the CON siRNA.
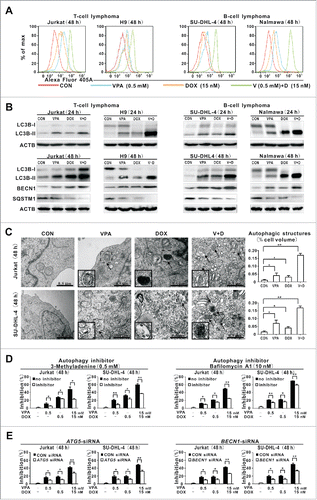
Figure 3. Valproic acid combined with doxorubicin induced PRKAA1/2 activation and MTOR inhibition though reducing cellular IP3 and mitochondrial calcium. (A) Phosphorylated and total protein expression of PRKAA1/2, MTOR, RPS6KB, EIF4EBP1, and phosphorylated ULK1 (p-ULK1 Ser555) detected by western blot in Jurkat and SU-DHL-4 cells treated with valproic acid (VPA, 0.5 mM) and/or doxorubicin (DOX, 15 nM) at 48 h. (B and C) Expression of HDAC1, HDAC3, p-PRKAA1/2, p-ULK1 Ser555 as well as LC3B-II and SQSTM1 by western blot in Jurkat cells transfected with HDAC1 and HDAC3 siRNA (B), or overexpression vectors (C), followed by treatment with VPA (0.5 mM) and DOX (15 nM) for 48 h. ACTB was used to monitor equivalent protein loading. (D) IP3 levels assessed by ELISA in Jurkat cells treated with VPA (0.5 mM) and/or DOX (15 nM) at 48 h. **P < 0.01,*P < 0.05 compared with the untreated cells. (E) IP3 levels in Jurkat cells transfected with HDAC1 and HDAC3 siRNA, followed by treatment with VPA (0.5 mM) and/or DOX (15 nM) at 48 h. (F) Representative immunofluorescence images of mitochondrial (green) and calcium (red) in Jurkat cells treated with VPA (0.5 mM) and/or DOX (15 nM) at 48 h. Cells were counterstained with DAPI (blue).
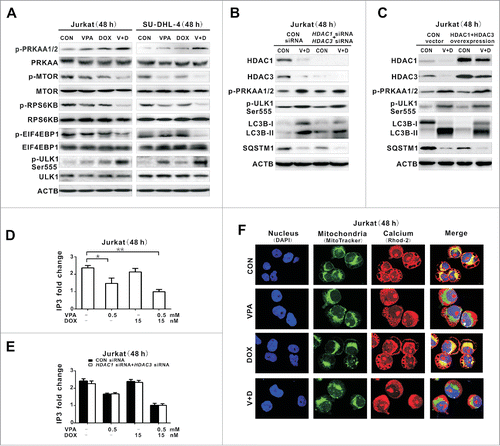
Figure 4. IP3-depleting effect of valproic acid mimicked by the IP3R inhibitor but counteracted by the calcium inhibitor. (A) IP3 levels assessed by ELISA in Jurkat cells treated with valproic acid (VPA, 0.5 mM), specific IP3R inhibitor Xestospongin C (XeC, 300 nM) or suberoylanilide hydroxamic acid (SAHA, 2 μM) at 48 h. **P < 0.01 compared with the CON (untreated) cells. (B) Representative immunofluorescence images of mitochondria (green) and calcium (red) in Jurkat cells treated with XeC (300 nM) or (SAHA, 2 μM) at 48 h. Cells were counterstained with DAPI (blue). (C) Expression of p-PRKAA1/2, p-ULK1 Ser555, LC3B-II and p-MTOR by western blot in Jurkat cells treated with XeC (300 nM) at 48 h. ACTB was used to monitor equivalent protein loading. (D) Representative immunofluorescence images of mitochondria (green) and calcium (red) in Jurkat cells transduced with the specific calcium inhibitor CALB1 vector and control vector, followed by treatment with VPA (0.5 mM) and DOX (15 nM) for 48 h. Cells were counterstained with DAPI (blue). (E) Expression of LC3B-II by western blot (Upper panel) and growth inhibition by MTT assay in Jurkat cells transduced with CALB1 overexpression vector and control vector, followed by treatment with VPA (0.5 mM) and DOX (15 nM) for 48 h. ACTB was used to assess equivalent protein loading. **, P < 0.01, *, P < 0.05 compared with the control vector.
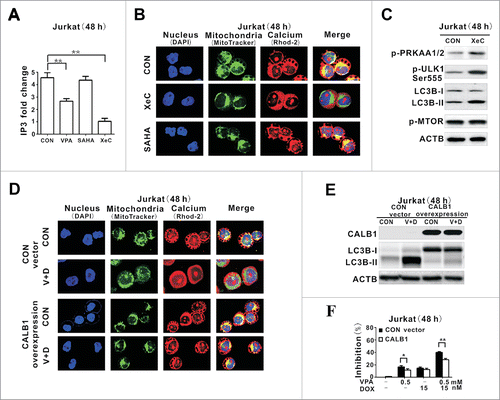
Figure 5. Valproic acid synergized with doxorubicin in murine xenograft lymphoma models. (A) Tumor volume of xenograft nude mice (injected subcutaneously with Jurkat and SU-DHL-4 cells) treated with valproic acid (VPA), doxorubicin (DOX), either alone or in combination. ***, P < 0.001 compared with the CON (untreated) group. (B) IP3 levels assessed by ELISA assay in xenograft tumors of the VPA group, DOX group and combination group. **, P < 0.01 compared with the CON (untreated) group. (C) Representative autophagic structures and quantitative data obtained by transmission electron microscopy in xenograft tumors of the VPA group, DOX group and combination group. Scale bars: 0.5 μm. **, P < 0.01, *, P < 0.05 compared with CON (untreated) group. (D) Immunohistochemical staining and semiquantitative data of p-PRKAA1/2 and BECN1 in xenograft tumors of the VPA group, DOX group and combination group. *, P < 0.05 compared with the CON (untreated) group.
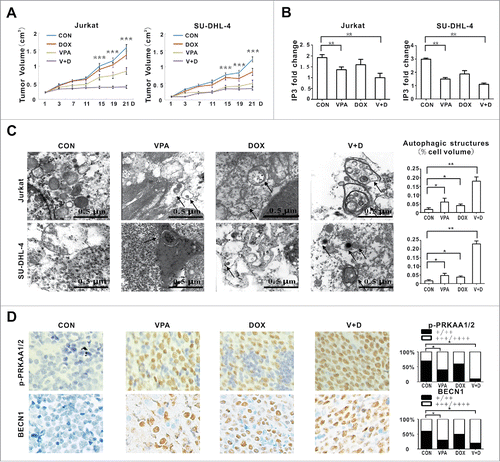
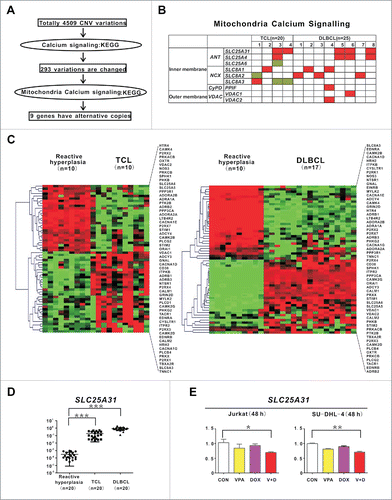
Figure 6. Genes involved in mitochondrial calcium transferring were altered in T- and B-cell lymphoma. (A) Schematic of CNV data analysis on tumor samples of 20 T-cell lymphoma (TCL) and 25 diffuse large B-cell lymphoma (DLBCL) patients. (B) Distribution of mitochondria calcium signaling gene aberrations in TCL (left panel) and DLBCL (right panel) cases. (C) Calcium signaling pathway revealed by gene expression profile in TCL and DLBCL cases. (D) Quantitative PCR results of SLC25A31 in TCL and DLBCL cases. ***, P < 0.001 compared with reactive hyperplasia. A relative quantification calculated using the ΔΔCT method based on the expression value of H9 cells. (E) Quantitative PCR results of SLC25A31 in Jurkat and SU-DHL-4 cells treated with valproic acid (VPA, 0.5 mM) and/or doxorubicin (DOX, 15 nM) at 48 h. **, P < 0.01, *, P < 0.05 compared with the CON (untreated) cells.