Figures & data
Figure 1. In mesothelioma cells, LC3 immunoblotting shows that autophagic flux differs between 2D and 3D cultures. (A) Cells were grown as monolayers (2D) or MCS (3D). Where indicated, the cells were exposed to 10 mM ammonium chloride (NH4+) for 8 h. LC3B expression was assessed by immunoblotting. As a loading control, filters were probed with anti TUBA/α-tubulin antibody. Band intensities were determined by densitometric analysis. The autophagic flux is expressed as a ratio of normalized LC3B-II band intensities after NH4+ to before NH4+ (NH4+:CTRL). A representative immunoblot of 3 independent experiments is shown, with ratios shown below. (B) Bars show the autophagic flux of 2D (light gray) or 3D (dark gray) cultures of the indicated cell lines. Data were obtained from 3 independent experiments, one of which is shown in (A). Asterisks indicate statistically significant differences of autophagic flux between 2D and 3D (P< 0.05). Error bars, SD. The mean LC3-II ratios in 3D are significantly higher in MCS-high autophagy than in MCS-low autophagy cell lines (P < 0.05).
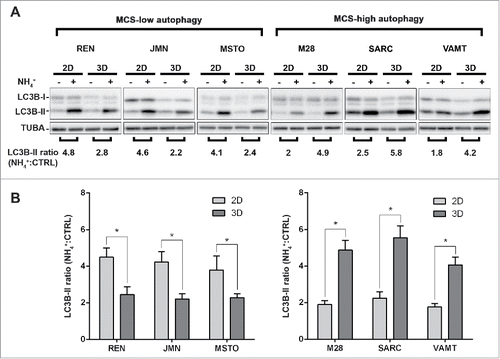
Figure 2. In mesothelioma cells, LC3 immunofluorescence confirms that autophagic flux differs between 2D and 3D cultures. (A) Cells were grown as in , trypsinized and cytospun on glass slides. Cells were then fixed and stained for LC3B (green) and nuclei (blue) and imaged by confocal microscopy. Arrowheads indicate LC3 puncta. Representative cells of 2 independent experiments are shown. Scale bars: 10 µm. (B) Bars show the ratio between LC3 puncta counted in cells grown in the presence or absence of 10 mM ammonium chloride (NH4+). Asterisks indicate significantly different LC3 puncta ratios between 2D and 3D (P< 0.05). Error bars, SD. The mean LC3 puncta ratios in 3D are also significantly higher in MCS-high autophagy than in MCS-low autophagy cell lines (P < 0.05).
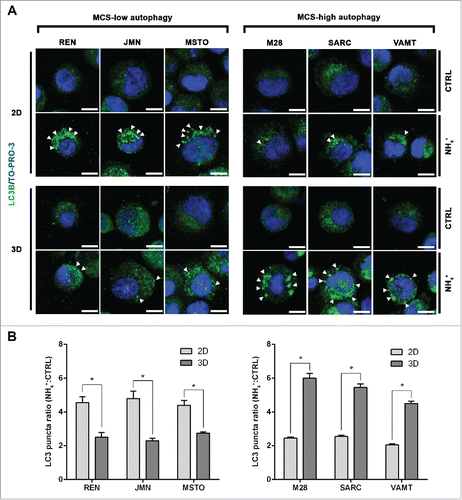
Figure 3. In mesothelioma cells, ATG13 puncta reflect the autophagic flux only in 3D. Mesothelioma cells were grown as monolayers (2D) on coverslips or as MCS (3D). Spheroid cells were trypsinized and cytospun on glass slides. Cells adherent on cover slips or glass slides were then fixed, stained for ATG13 (green) and nuclei (blue), and imaged by confocal microscopy. Representative ATG13 puncta are indicated by arrowheads. Scale bars: 10 µm.
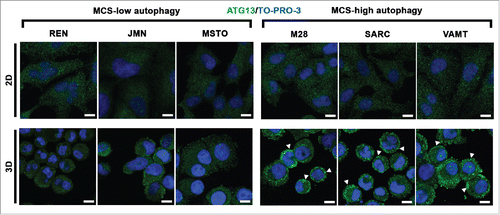
Figure 4. In tumor fragment spheroids, LC3 immunofluorescence indicates low or high levels of autophagy. TFS were generated from tumor biopsies obtained from 25 chemonaive MPM patients and grown in the presence or absence of ammonium chloride (NH4+) for 12 h. TFS were then fixed, embedded in paraffin, stained for LC3B (green), KRT/cytokeratin to identify mesothelioma cells (red), and nuclei (blue), and imaged by confocal microscopy. (A) Bars represent the mean percentage of LC3-positive MPM cells measured in TFS grown in the presence (gray bars) or absence (white bars) of NH4+. Error bars, SEM (B) Representative images of TFS with either low (TFS #8) or high (TFS #2) autophagy levels are shown, with the percentage of mesothelioma cells with LC3 puncta (LC3-positive MPM cells) indicated in parentheses. Zoom-in view of the region in the dashed box shows representative cells with LC3 puncta (arrowhead). Scale bars: 10 µm.
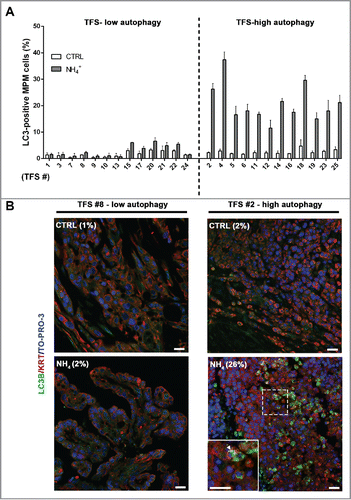
Table 1. In tumor fragment spheroids, LC3-positivity is shown at baseline and after ammonium chloride (NH4+) treatment. The percentages of LC3-positive MPM cells are shown for low (A) or high (B) autophagy TFS grown in the presence (NH4+) or absence (CTRL) of ammonium chloride. For each TFS, data are expressed as mean percentages. Parentheses, SEM. The difference in the percentage of LC3B-positive MPM cells following exposure to ammonium chloride (NH4+-CTRL) is also shown for each TFS.
Figure 5. In tumor fragment spheroids, ATG13 puncta analysis reflects the low or high levels of autophagy. The TFS shown in and not exposed to ammonium chloride (NH4+) were fixed, embedded in paraffin, stained for ATG13 (green), KRT/cytokeratin to identify mesothelioma cells (red) and nuclei (blue), and imaged by confocal microscopy. (A) Bars represent the mean percentage of ATG13-positive MPM cells for the TFS determined in to be low or high ATG. Error bars, SEM (B) Representative images of TFS with either low autophagy (TFS #8) or high autophagy (TFS #2) levels are shown, with the percentage of mesothelioma cells with ATG13 puncta (ATG13-positive MPM cells) indicated in parentheses. Zoom-in view of the region in the dashed box shows representative cells with ATG13 puncta (arrowhead). Scale bars: 10 µm.
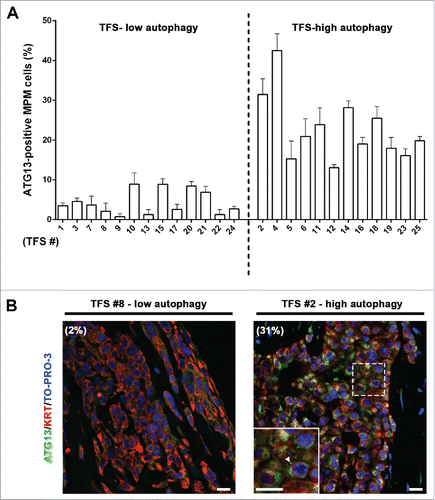
Figure 6. In tumor fragment spheroids, autophagy initiation correlates with the autophagic flux. (A) TFS exposed to NH4+ were stained for LC3 and the same TFS not exposed to NH4+ were stained for ATG13; both were stained for KRT/cytokeratin to identify the mesothelioma cells. The correlation plot of the percentages of LC3-positive MPM cells (y axis, TFS grown in the presence of NH4+) relative to that of ATG13-positive MPM cells (x axis, TFS grown without NH4+) is shown. Arrows identify the position of the representative low autophagy TFS (#8, black circle) and high autophagy TFS (#2, gray circle), previously shown on . Spearman rank correlation (rs), 0.8997; P (2-tailed)< 0.0001. (B) TFS grown in the presence or absence of NH4+ for 12 h were stained for ATG13 (green), LC3A/B (red), and nuclei (blue) and imaged by confocal microscopy. ATG13 and LC3A/B double immunostaining images of a representative TFS with high autophagy levels (TFS #2) grown in the presence (NH4+) or absence of the lysosomal inhibitor (CTRL) are shown. Zoom-in views of the regions in the dashed boxes are shown for representative cells. Scale bars: 10 µm.
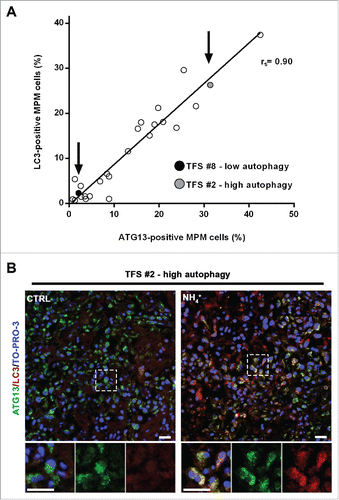
Figure 7. In tumor fragment spheroids, autophagy initiation correlates with that of the original tumors. Fixed samples of the original tumors used to generate the analyzed 25 TFS were stained for ATG13 (green), KRT/cytokeratin (red) and nuclei (blue) and imaged by confocal microscopy. (A) The correlation plot of the percentages of ATG13-positive MPM cells measured in formalin-fixed MPM clinical samples (y axis) relative to those measured in the respective TFS (x axis) is shown. Arrows identify the position of the representative low autophagy (MPM or TFS #8, black circle) and high autophagy (MPM or TFS #2, gray circle) tumor, previously shown in . Spearman rank correlation (rs), 0.9253; P (2-tailed)< 0.0001. (B) Representative images of MPM sections corresponding to TFS with either low autophagy (MPM #8) or high autophagy (MPM #2) levels are shown, with the percentage of mesothelioma cells with ATG13 puncta (ATG13-positive MPM cells) indicated in parentheses. Zoom-in view of the region in the dashed box shows representative cells with ATG13 puncta (arrowhead). Scale bars: 10 µm.
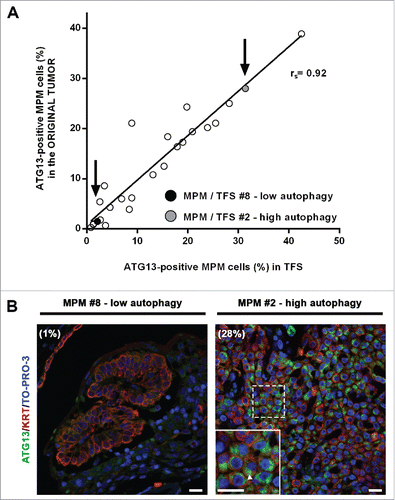
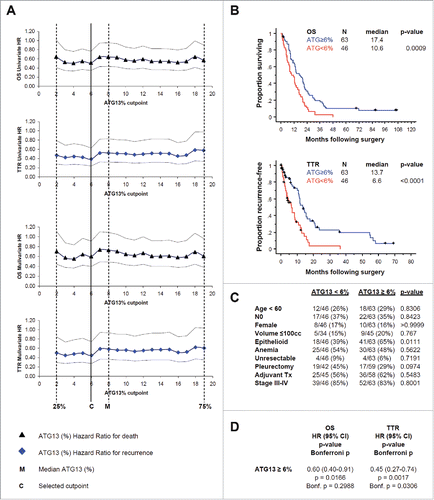
Figure 8. In formalin-fixed tissue microarray with tumor from 109 patients, ATG13 correlates with clinical outcome. (A) Cox regression analyses, univariate or with correction for the tumor histology, for death (OS) or recurrence (TTR) are shown. Hazard ratios (HR) with 95% confidence intervals are shown at 18 candidate ATG13% cutpoints. M, median percentage of ATG13-positive MPM cells. C, selected cutpoint (6%). (B) Kaplan-Meier curves are shown for overall survival (OS) and time to recurrence (TTR) at the 6% ATG13-positive MPM cells cutpoint selected in (A). (C) Correlation analysis of autophagy levels and known prognostic factors. ATG13 did not correlate with age, nodal status, gender or tumor volume, anemia, resectability, surgical procedure, adjuvant therapy, or TNM stage; high ATG13 positivity was more common in epithelioid tumors. (D) Multivariate analysis adjusted for the effect of epithelioid histology shows that ATG13 positivity remains significantly prognostic for TTR (P = 0.0017) and for OS (P = 0.0166), even after adjusting for the effect of histology. When conservatively corrected for the repeated measures required to establish the cutpoint, ATG13 positivity remains significantly prognostic for TTR (Bonferroni corrected P = 0.0306) but not for OS (corrected P = 0.2988).