Figures & data
Figure 1. Activation of CAR3 attenuates myasthenia in EAMG mice. Female C57BL/6 mice (6- to 8-wk old) were immunized s.c. with Torpedo californica chrn in complete Freund's adjuvant, and were boosted 4 wk later. After 4 more wk, the mice were anesthesized with sodium pentobarbital, fixed and connected to the electrodes of an electromyography machine. Compound muscle action potential in response to repetitive stimulation of the nerve at 3-Hz was recorded (A), and evoked action potential decrements were displayed (B). (C) Blood was collected from mice tail veins and serum was prepared and subjected to ELISA assay for serum anti-CHRN. (D) Paw grip endurance of EAMG mice with or without treatment with AP as per the protocol in (E). (E) Schematic plan of treatment for the mice. EAMG mice were established as mentioned in (A), other than 1-(2-aminoethyl) piperazine (AP, 2 μg/ml) was given in the drinking water from the second immunization (boost). (F) Clinical score of EAMG mice with or without treatment with AP. The images shown are representative of 3 independent experiments. Data are mean ± SEM of 3 independent experiments (B, C, and E), *p < 0.05.
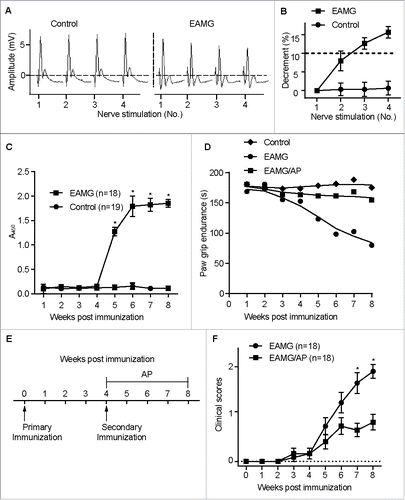
Figure 2. CAR3 regulates CHRN degradation in muscle cells. Gastrocnemius from control, EAMG and EAMG mice treated with AP was homogenized in lysis buffer containing 1% NP-40 and subject to SDS-PAGE and immunoblot analysis with anti-CAR3 (A) or anti-CHRN (C) antibodies. Densitometry quantification of total CAR3 (B) or CHRN (D) over ACTB/β-actin was performed using ImageJ software. (E) Total RNA was extracted from muscles, and quantitative reverse-transcribed PCR was performed using Chrna1-specific primer pairs. The results represent the mean ± SEM of 3 independent experiments (B, D, and E). *p < 0.05.
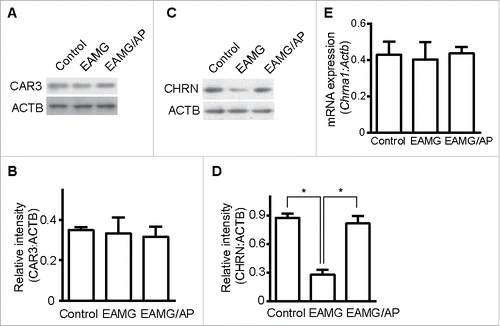
Figure 3. Endocytosis of CHRN is mediated via a lipid raft-mediated pathway. (A) C2C12 cells were transfected with a plasmid encoding RAC1T17N, or pretreated with Dynasore (50 μM) or MβCD (5 μM) before incubation with Alexa Fluor 488-conjugated anti-CHRN antibody (mAb210) at 4°C for 1 h, then the cultures were switched to 37°C for different times to induce CHRN endocytosis. After acidic washes, the cells were fixed and analyzed using flow cytometry. (B) C2C12 cells were transfected with a plasmid encoding RAC1T17N, or pretreated with Dynasore (50 μM) or MβCD (5 μM) before being incubated with biotin-CHRN antibody (mAb210) at 4°C for 1 h, and then switched to 37°C for different times to induce CHRN endocytosis. After acidic washes, the cell lysate was prepared and subjected to SDS-PAGE and blotted with streptavidin-HRP. Shown is a representative image of 3 experiments (B), and the quantitative data are presented as the mean ± SEM of 3 experiments (A). *p < 0.05, between the MβCD group and the control group; # p < 0.05, between the RAC1T17N group and the control group.
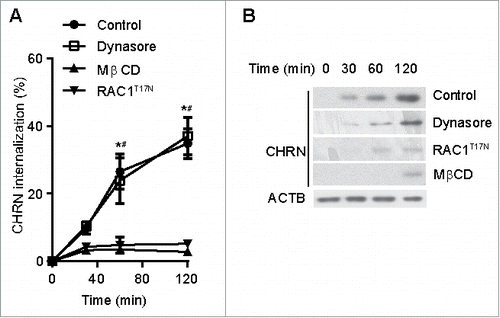
Figure 4. CAR3 specifically regulates CHRN endocytosis. (A) C2C12 cells treated with AP (2 μg/ml), TFMS (2 mM) or AZA (1 mM) for 6 h, were lysed and subject to SDS-PAGE followed by immunoblot analysis using CAR3, CAR2 or ACTB antibodies. (B) C2C12 cells were treated with or without AP (2 μg/ml), TFMS (2 mM) or AZA (1 mM) for 6 h, followed by incubation with CHRN antibody (mAb210) at 4°C for 1 h, and then switched to 37°C for different times to induce CHRN endocytosis. After acidic washes, the cells were fixed and analyzed with flow cytometry. (C) C2C12 cells treated without or with AZA (1 mM), AP (2 μg/ml), TFMS (2 mM) for 6 h, and then labeled with biotin-CHRN antibody (mAb210). After further culture for 2 h, C2C12 cells were washed with acidic buffer, lysed and analyzed using SDS-PAGE and blotted with streptavidin-HRP. (D) The band densitometry was quantified using ImageJ software. Shown is a representative image of three experiments (A and C), and the quantitative data are presented as the mean ± SEM of 3 experiments (B). *p < 0.05, between the MβCD group and the control group; #p < 0.05, between the AP group and the control group; &p < 0.05, between the TFMS group and the control group.
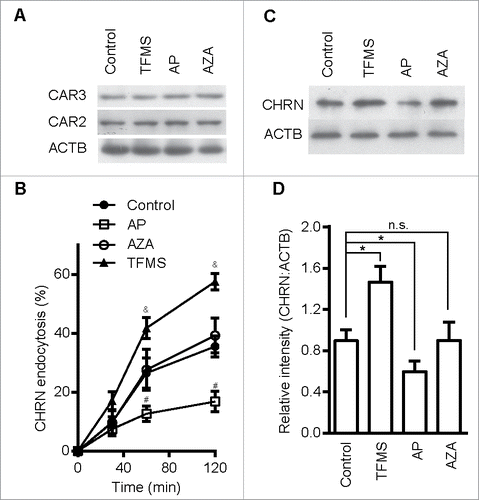
Figure 5. CAR3 suppression leads to enhanced CHRN endocytosis. C2C12 cells were transiently transfected with the indicated plasmids or specific siRNA using Lipofectamine 3000. 48 h later, the cells were either lysed and subjected to SDS-PAGE and immunoblotted with CAR2 or CAR3 antibody (A); or incubated with CHRN antibody (mAb210) at 4°C for 1 h, switched to 37°C for different times to induce CHRN endocytosis, subjected to acidic washes, then fixed and analyzed with flow cytometry (B); or were lysed and subjected to SDS-PAGE and immunoblotted with CHRN antibody (C). (D) The band densitometry was quantified using ImageJ software. (E) C2C12 cells were lysed and followed by immunoprecipitation with the indicated antibody, and then blotted with the specified antibodies. Data are mean ± SEM of 3 independent experiments, *p < 0.05, between the CAR3 group and the control group; #p < 0.05, between the siCar3 group and the siScram group.
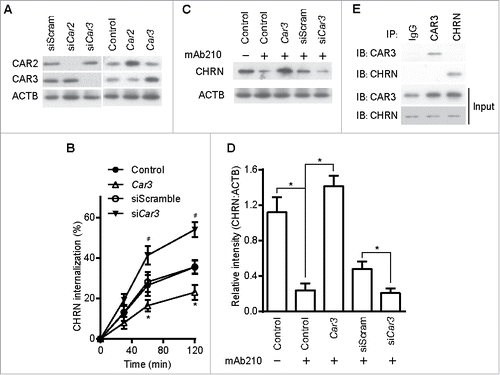
Figure 6. CAR3 suppresses endocytosis via a macroautophagy-mediated pathway. (A) Gastrocnemius from mice was homogenized in lysis buffer containing 1% NP-40, subject to SDS-PAGE and immunoblot analysis with the indicated antibody. Densitometric quantification of the indicated proteins over ACTB using ImageJ (right panel). (B) Gastrocnemius fixed, frozen sectioned, and permeabilized. After being blocked with 20% goat serum, the sections were incubated with anti-MAP1LC3A/B antibody and the appropriate Alexa Fluor 488-conjugated secondary antibody. After washing with PBS, the sections were examined with a confocal microscope and images were captured (left panel). Scale bar: 20 µm. MAP1LC3A/B-positive puncta were counted, and at least 50 cells were quantified. Puncta per cell are shown as mean ± SEM of 3 independent experiments (right panel). (C) C2C12 cells were transiently transfected with siScram or siAtg7 using Lipofectamine 3000. Forty-eight h later, the cells were either lysed and subjected to SDS-PAGE and immunoblot analysis with CHRN antibody (total CHRN, CHRN-t), or labeled with biotin-CHRN antibody (mAb210) and after 2 h the cells were washed with acidic buffer, lysed and analyzed by SDS-PAGE and immunblot analysis with streptavidin-HRP for endocytosed CHRN (CHRN-e). *p < 0.05, compared with the control group. Data are mean ± SEM of 3 independent experiments.
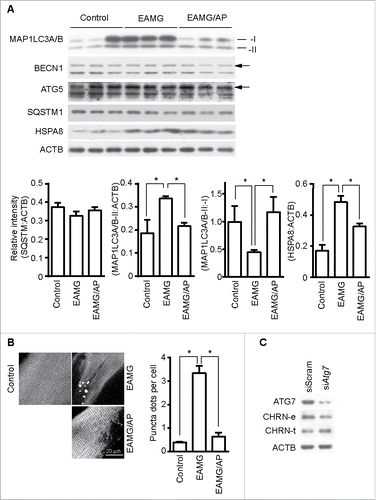
Figure 7. CAR3 suppresses endocytosis by repressing chaperone-assisted selective autophagy. (A) C2C12 cells were lysed, immunoprecipitated with the indicated antibody, then blotted with the specified antibodies. (B and C) C2C12 cells were lysed, followed by immunoprecipitation with the indicated antibody, and then blotted with the specified antibodies. (D) C2C12 cells were transiently transfected with specific siRNA using Lipofectamine 3000. Forty-eight h later, the cells were treated with TFMS (2 mM) for 6 h. Cell lysates from these cells were separated by SDS-PAGE and analyzed by immunoblotting with the indicated antibodies. (E) C2C12 cells were transiently transfected with specific siRNA against Car3 using Lipofectamine 3000. Forty-eight h later, the cells with treated with TFMS (2 mM) for 6 h followed by incubation with CHRN antibody (mAb210) at 4°C for 1 h, and then switched to 37°C for different times to induce CHRN endocytosis. After acidic washes, the cells were fixed and analyzed with flow cytometry. (F) C2C12 cells were transiently transfected with siScram or siBag3 using Lipofectamine 3000. Forty-eight h later, the cells were lysed and subjected to SDS-PAGE and analyzed by immunoblotting with anti-BAG3 antibody. (G) C2C12 cells were transiently transfected with specific siRNA (siBag3) using Lipofectamine 3000. Forty-eight h later, either control C2C12 cells or siBag3-transfected cells were treated with vehicle or TFMS (2 mM) for 6 h followed by incubation with CHRN antibody (mAb210) at 4°C for 1 h, and then switched to 37°C for different times to induce CHRN endocytosis. After acidic washes, the cells were fixed and analyzed with flow cytometry. All immunoblotting and immunoprecipitation studies were performed 3 times. *p < 0.05, compared with the control group. Data are mean ± SEM of 3 independent experiments.
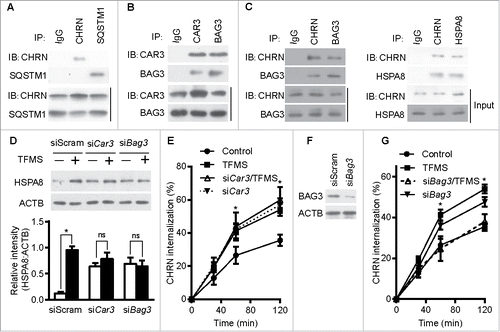
Figure 8. CAR3 regulates endocytosis of CHRN through ER stress. (A) Gastrocnemius from mice was homogenized in lysis buffer containing 1% NP-40, subject to SDS-PAGE and immunoblot analysis with the indicated antibody. (B) Densitometric quantification of the indicated proteins over ACTB was performed using ImageJ. (C) C2C12 cells were transiently transfected with the indicated plasmids using Lipofectamine 3000. Forty-eight h later, the cells were treated with tunicamycin (TM; 2 μM) for 12 h, followed by incubation with CHRN antibody (mAb210) at 4°C for 1 h, and then switched to 37°C for different times to induce CHRN endocytosis. After acidic washes, the cells were fixed and analyzed with flow cytometry. *p < 0.05, between the TM group and the control group; #p < 0.05, between the TM group and the Car3-TM group. (D) C2C12 cells were transiently transfected with the indicated specific siRNA using Lipofectamine 3000. Forty-eight h later, the cells with treated with PBA (2 μM) for 12 h followed by incubation with CHRN antibody (mAb210) at 4°C for 1 h, and then switched to 37°C for different times to induce CHRN endocytosis. After acidic washes, the cells were fixed and analyzed with flow cytometry. *p < 0.05, between siCar3 group and control group; #p < 0.05, between siCar3 group and siCar3-PBA group. (E) C2C12 cells were transiently transfected with siScramble or specific siRNA (siBag3) using Lipofectamine 3000. Forty-eight h later, the cells with treated with TFMS (2 mM) for 6 h followed by SDS-PAGE and immunoblot analysis with the indicated antibody. Data are mean ± SEM of 3 independent experiments (B, C, and D).
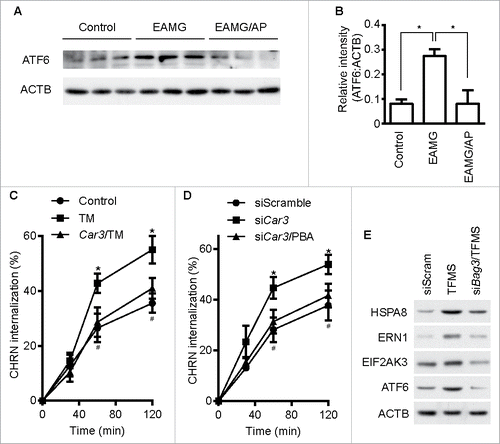
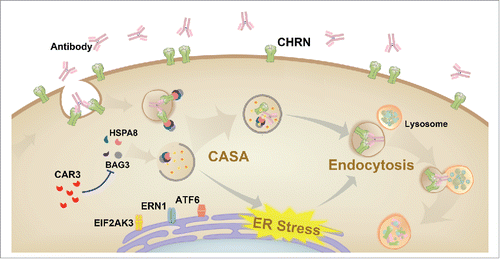
Figure 9. Schematic diagram of the mechanisms of CAR3 in maintaining homeostasis of CHRN. CAR3 interacts with BAG3 to inhibit chaperone-assisted selective autophagy (CASA), which regulates endocytosis of CHRN through interaction between BAG3-HSPA8 and CHRN. CAR3 also reduces ER stress, preventing excessive degradation of CHRN in muscle cells.