Figures & data
Figure 1. ULK1 and ULK2 are required for axonal guidance in the forebrain. (A) Brain sections of E18.5 WT (1, 2, 3), ulk1-KO (4, 5, 6), ulk2-KO (7, 8, 9), and ulk1/2-DKO (10, 11, 12) mice were stained with antibodies against the general axonal marker CHL1 (pseudocolored green). The callosal axons are heavily overfasciculated, and the corpus callosum is thinner in ulk1/2-DKO mice (10′). The anterior commissure fails to develop (11′). CTAs and TCAs progress abnormally as they cross the PSPB (12′). The ulk1/2-DKO axons are highly disorganized and extend aberrantly towards the external capsule (arrows in 2–3, 5–6, 8–9, and 11–12). The CTAs and TCAs are also overfasciculated. (B) Quantification of the dorsoventral width of the corpus callosum of control (n = 5) and ulk1/2-DKO mice (n = 5) at E18.5. (C-D) The axon pathfinding defects in ulk1/2-DKO mice are detected as early as E14.5 (dashed lines). Abbreviations: AC, anterior commissure; CC, corpus callosum; CTAs, corticothalamic axons; EC, external capsule; HC, hippocampal commissure; CHL1, cell adhesion molecule L1-like; PSPB, pallial-subpallial boundary; TCAs, thalamocortical axons. Scale bars: 500 µm in (A1-A12, and C); 250 µm in (A1′-A12′). **P <0.01 in (B and D).
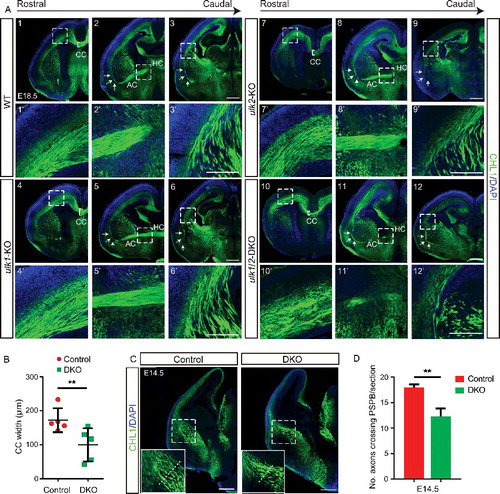
Figure 2. ULK1 and ULK2 are not required for autophagy-mediated turnover of ubiquitinated protein in the cerebral cortex. (A) Immunoprecipitation of ULK1 by using extracts prepared from the cortex of P0 mice and performed in the presence or absence of epitope-specific blocking peptides. The interaction between ULK1, ATG13, and RB1CC1 was detected in extracts prepared from P0 cortex, whereas that between ULK1 and SEC16A was detected in extracts prepared from P0 cortex in the presence or absence of RB1CC1. (B) Extracts prepared from the cortex of E18.5 control and ulk1/2-DKO mice were immunoblotted with antibodies against autophagy proteins, including SQSTM1, LC3B, ATG13, RB1CC1, and ATG14. (C) Quantification of the SQSTM1 level in the E18.5 cortex of control (n = 3) and ulk1/2-DKO mice (n = 3). (D and E) Immunoblot and immunostaining analyses of the cortex from control, Ulk1/2-cDKO, Atg7-cKO, and Rb1cc1-cKO mice at P0 by using antibodies against SQSTM1. (F) SQSTM1 (pseudocolored red) and ubiquitin (pseudocolored green) staining of the cortex from control, Ulk1/2-cDKO, Atg7-cKO, and Rb1cc1-cKO mice at P21. Sections were counterstained with DAPI (pseudocolored blue). Abbreviations: BP, blocking peptides; IP, immunoprecipitation; ns, not significant. Scale bars: 50 µm.
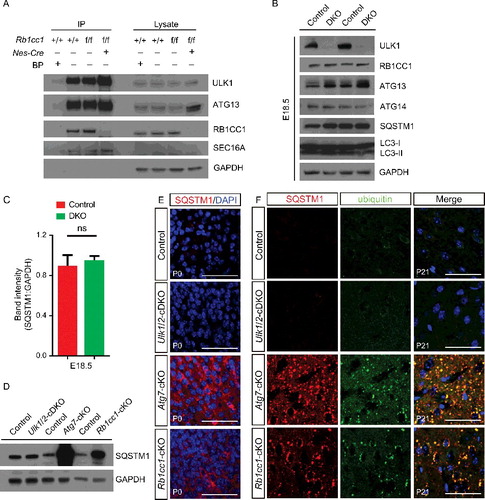
Figure 3. RB1CC1 and ATG7 are not required for axon guidance in the forebrain. CHL1 (green) staining of serial sections of P1 Ulk1/2-cDKO brains reveals abnormally fasciculated axons, including the CTAs and TCAs (inset), corpus callosum dysgenesis, and anterior commissure hypoplasia. These axon guidance abnormalities are similar to those identified in the ulk1/2-DKO mice and are not observed in autophagy-deficient Atg7-cKO or Rb1cc1-cKO mice. Abbreviations: AC, anterior commissure; CC, corpus callosum; EC, external capsule; HC, hippocampal commisure; IC, internal capsule; CHL1, cell adhesion molecule L1-like; Pb, Probst bundle. Scale bars: 500 µm.
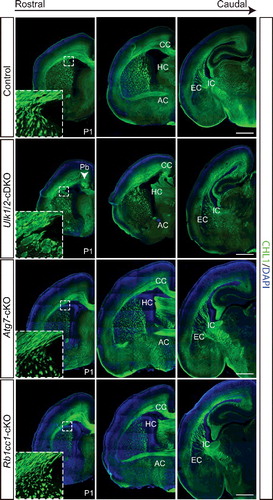
Figure 4. ULK1 and ULK2 are not required for maintaining the neural stem cells in the hippocampus. (A) Immunostaining of markers for neuronal stem cells (SOX2; green), mitotic cells (MKI67/KI67; red), and mature neurons (RBFOX3/NEUN; blue) in the dentate gyrus of adult control and Ulk1/2-cDKO mice. (B) The mean number ± SEM of the 3 cell types in the dentate gyrus showed no statistical significance between controls (n = 3) and Ulk1/2-cDKO (n = 3) mice. Abbreviations: GZ, granule zone; ns, not significant; SGZ, subgranular zone.
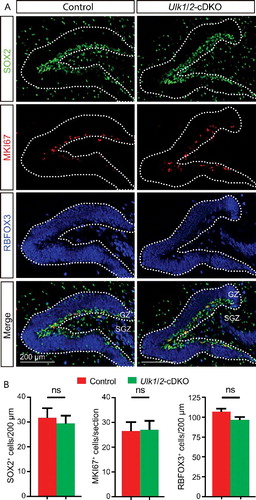
Figure 5. The cortical layers of the ulk1/2-DKO brain are grossly normal. (A) Nissl staining (A1-A2) and expression of the callosal neuron marker SATB2 (red), layer V marker BCL11B/CTIP2 (blue), and layer VI marker TBR1 (green) in control and ulk1/2-DKO cortices at E18.5 (A3-A4), and (B). The quantifications of SATB2+, BCL11B/CTIP2+, and TBR1+ cells in layers II-IV, V, and VI, respectively, are not statistically significant (ns), indicating that Ulk1/2 are not required for neurogenesis. (C and D) Staining of cleaved CASP3 (green) in E18.5 control and ulk1/2-DKO cortices (C) and the number of cleaved-CASP3+ cells per section (D) was quantified (n = 3 for control and ulk1/2-DKO mice). Arrows in (C) point to the sparse apoptosis present in both the control and ulk1/2-DKO cortices. Scale bars: 50 µm in (A1-A2, A3′-A4′), and C; 500 µm in (A3-A4).
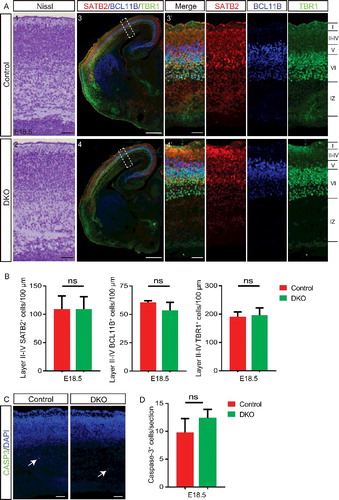
Figure 6. ULK1 and ULK2 regulate development of the corpus callosum. (A and B) Dil was placed in the parietal cortex of E18.5 ulk1/2-DKO (n = 2) and control (n = 2) mice to trace the midline crossing of callosal axons. Representative sections (A) and quantification (B) highlight the dramatic reduction in fluorescence intensity in the contralateral cortex (rostral and caudal levels) of ulk1/2-DKO mice (asterisks in A). (C) Luxol Fast Blue (LFB) and Nissl staining reveals persistent anomalies of the corpus callosum in the adult Ulk1/2-cDKO mice. (D) The dorsoventral width of the corpus callosum of adult control (n = 3) and Ulk1/2-cDKO mice (n = 3) was quantified, and the average width of each mutant was normalized to that of its corresponding control. There is a significant reduction in the width of the corpus callosum in Ulk1/2-cDKO mice relative to that in controls. (E) Representative brain sections from E18.5 ulk1/2-DKO mice, P0 Ulk1/2-cDKO mice, and age-matched controls. The midline glial structure developed properly. (F) CALR (calreticulin) immunostaining (green) of the glutamatergic guidepost neurons in control and ulk1/2-DKO brains at E15.5 and E18.5 showed that the production of those neurons is unaltered in the Ulk1/2-deficient mice. Abbreviations: CC, corpus callosum; GW, glial wedge; IGG, indusium griseum glia, MZG, midline zipper glia; Pb, Probst bundle. Scale bars: 500 µm in (A1-A4, and C1-C2); 100 µm in (A1′-A4′, C1′-C2′, and E-F). **P <0.01 in (B and D).
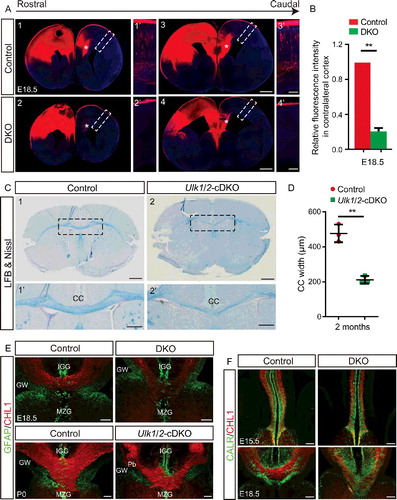
Figure 7. ULK1 and ULK2 are required for proper organization of the somatosensory cortex. (A) Representative images of fluorescence signal from Dil crystals embedded in the somatosensory cortex reveals the disorganized CTA projections crossing the PSPB in E18.5 ulk1/2-DKO brains. These Ulk1/2-deficient axons, despite their disorganization and abnormal fasciculation, ultimately reach their targeted destination and innervate the dTh (A2 and A4). (B) Normal SLC17A6 staining (red) and cytochrome oxidase staining in the dTh in the ulk1/2-DKO brains at P21 suggests proper cortical innervation of that structure. (C) Dil tracing shows the severely disorganized TCA projections in the ulk1/2-DKO brains. (D) Representative images of CALR (calreticulin) staining (green) reveal a dramatic reduction in the number of TCA projections reaching the cortex in E18.5 ulk1/2-DKO brains. (E) Representative images of sections immunostained using antibodies against the cortical barrel markers SLC6A4 (green) and SLC17A6 (red). Arrows indicate barrels. (F) The number of barrel units per section was quantified from P7 control (n = 3) and Ulk1/2-cDKO (n = 3) brains. **P <0.01. (G) Cytochrome oxidase staining reveals the disorganization of the somatosensory barrel cortex in P7 Ulk1/2-cDKO brains. Abbreviations: A, anterior; COX, cytochrome oxidase; dTh, dorsal thalamus; M, medial; VPL, ventral posterolateral nucleus; VPM, ventral posteromedial nucleus. Scale bars: 100 µm in (A1′-A3′, B, C2′-C4′, D1′-D2′, E, and G); 500 µm in (A1-A4, C1-C4, and D1-D2).
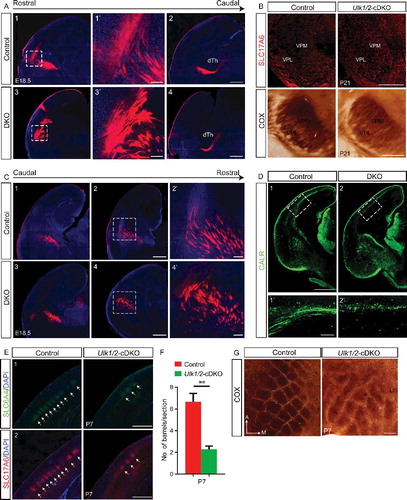
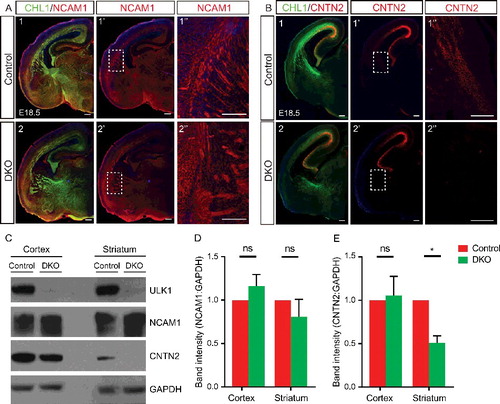
Figure 8. Abnormal axonal fasciculation in the Ulk1/2-deficient animals is associated with mislocalization of CNTN2 in the projection neurons. (A) Neuronal cell adhesion molecule distribution was unaltered in the ulk1/2-DKO brains compared to that in the controls. (B) The intensity of CNTN2 immunostaining (red) was dramatically decreased in distal CTAs of the ulk1/2-DKO brain at E18.5. All of the sections were counterstained with CHL1 cell adhesion molecule (green). (C) Western blot analyses of the extracts prepared from the cortex (proximal CTAs) and striatum (distal CTAs) confirmed normal expression of neuronal cell adhesion molecule but significantly decreased CNTN2 levels in the striatum. (D and E) Quantification of the NCAM1 and CNTN2 levels in control and ulk1/2-DKO cortex and striatum. Abbreviations: Cnt, controls; NCAM1, neuronal cell adhesion molecule 1; ns, not significant.*P < 0.05. Scale bars: 200 µm.