Figures & data
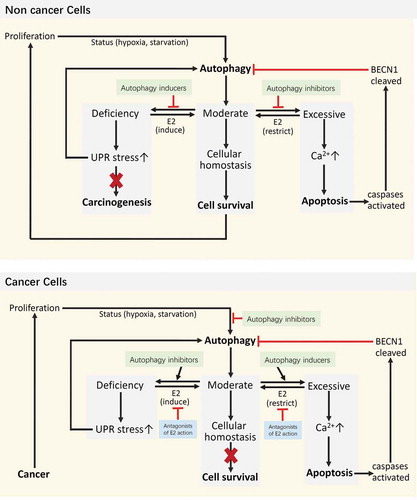
Table 1. The regulation of E2 on autophagy.
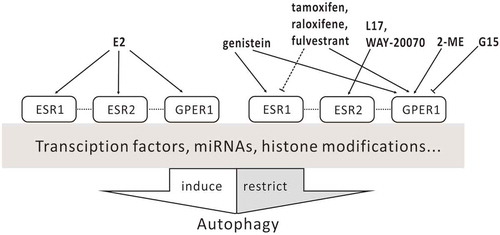
Table 2. The regulation of autophagy by ESR ligands a.
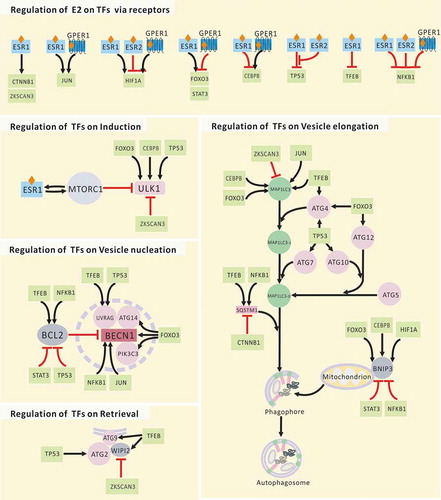
Table 3. Autophagy proteins regulated by E2 via TFs.