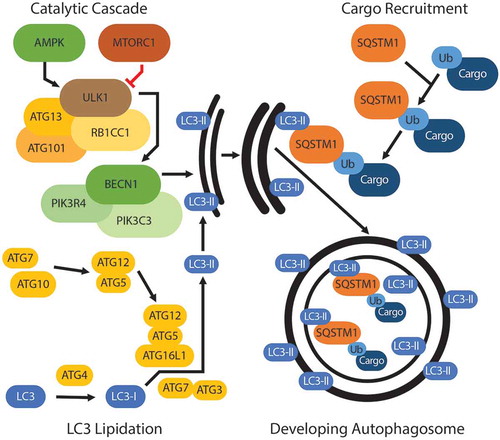
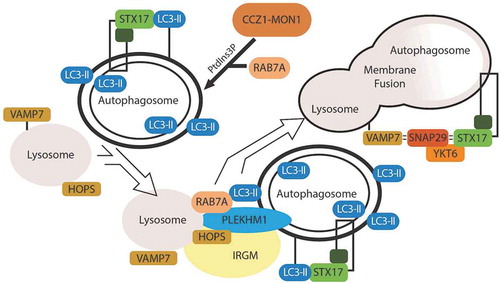
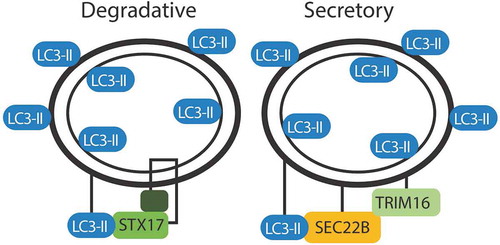
Figures & data
Table 1. Secreted entities regulated by autophagy-dependent secretion.