Figures & data
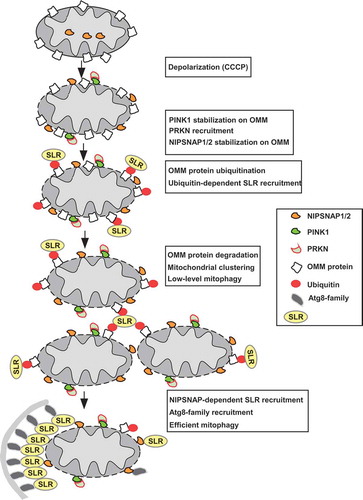
Figure 1. Working model of PINK1-PRKN-dependent mitophagy illustrating the role of NIPSNAP1 and NIPSNAP2 as ‘eat me’ signals for recruiting autophagy receptors. Mitochondrial damage results in stabilization of PINK1 and recruitment of PRKN. PRKN-dependent ubiquitination of OMM protein leads to proteasome-dependent degradation of OMM proteins and ubiquitin-dependent recruitment of the SLRs. This is accompanied by localization of NIPSNAP1 and NIPSNAP2 to the OMM. The result is the sustained recruitment of the SLRs and Atg8-family proteins to mediate robust mitophagy. The PINK1-PRKN pathway contains important loops; ubiquitination of OMM proteins leads to both the recruitment of the SLRs and proteasomal degradation of the ubiquitinated OMM proteins. The ubiquitin-dependent recruitment of SLRs leads to mitochondrial clustering and low-level mitophagy. Continuous damage and increased degradation of the OMM proteins prime the mitochondria, allowing the OMM-localized NIPSNAP1 and NIPSNAP2 to act as ‘eat-me’ signals, recruiting SLRs and Atg8-family proteins to mediate robust mitophagy.