Figures & data
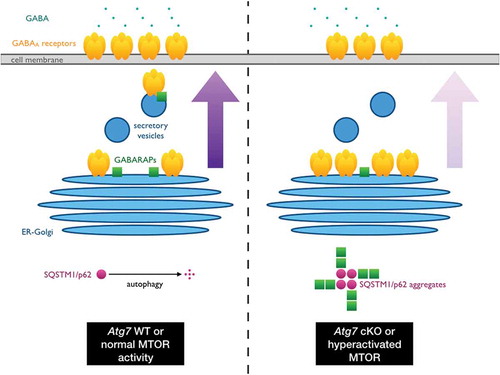
Figure 1. SQSTM1/p62 aggregates link MTOR and GABA signaling. Under normal physiological conditions, GABARAP family proteins mediate the trafficking of GABAA receptors from the ER-Golgi to the cell surface of neurons to receive inhibitory signals from GABAergic interneurons. However, in autophagy-deficient or MTOR-hyperactivated neurons, SQSTM1/p62 accumulates and forms protein aggregates, which in turn sequester GABARAPs. As the conformational status and subcellular localization of GABARAPs are altered by this co-aggregation event, their ability to mediate GABAA receptor trafficking is reduced and thus the affected neurons become hyperactive due to a loss of inhibitory inputs. The resulting shift in excitatory-inhibitory balance in turn contributes to the autistic-like behavioral abnormalities exhibited by autophagy-deficient mice and mouse models or ASD patients with hyperactivated MTOR signaling due to mutations in upstream regulatory molecules such as PTEN and TSC1/2.