Figures & data
Figure 1. Core mechanism of ferroptosis. System xc−-mediated cystine uptake and subsequent GSH production and GPX4 activation play a central role in protecting cells from ferroptosis. Alternatively, AIFM2 inhibits ferroptosis by catalyzing the production of CoQ10H2 from CoQ10 or promoting ESCRT-III–dependent membrane repair. Ferritinophagy and lipophagy provide the substrates Fe2+ and FA, respectively, for the execution of lipid peroxidation during ferroptosis. This process is accompanied by ACSL4-catalyzed arachidonic acid-CoA formation, followed by LPCAT3-mediated arachidonic acid-CoA esterification to phospholipids (PL). Several ROS-producing enzymes (e.g., ALOXs, POR, and NOXs) are also involved in the induction of lipid peroxidation. The red text and blue text indicate ferroptosis inducers and inhibitors, respectively. Abbreviations: 2ME, beta-mercaptoethanol; AA, arachidonic acid; ACLS4, acyl-CoA synthetase long chain family member 4; Ada, adrenic acid; AIFM2, apoptosis inducing factor mitochondria associated 2; ALOXs, lipoxygenases; ANGPTL4, angiopoietin like 4; CoA, coenzyme A; CoQ10, coenzyme Q10; CoQ10H2, ubiquinol; DPP4, dipeptidyl peptidase 4; EMP1, epithelial membrane protein 1; ESCRT-III, endosomal sorting complex required for transport-III; FA, fatty acid; G6PD, glucose-6-phosphate dehydrogenase; GCL, glutamate-cysteine ligase; GPX4, glutathione peroxidase 4; GSH, glutathione; GSR, glutathione-disulfide reductase; GSS, glutathione synthetase; GSSG, oxidized glutathione; H2O2, hydrogen peroxide; HDDC3, HD domain containing 3; LPCAT3, lysophosphatidylcholine acyltransferase 3; NAC, N-acetyl cysteine; NADK, NAD kinase; NCOA4, nuclear receptor coactivator 4; NOXs, NADPH oxidases; PGD, phosphogluconate dehydrogenase; PL, phospholipid; PLOOH, phospholipid hydroperoxides; POR, cytochrome p450 oxidoreductase; PPP, pentose phosphate pathway; RAB7A, RAB7A, member RAS oncogene family; RTAs, radical-trapping antioxidants; Se, selenium; t-BuOOH, tert-butyl hydroperoxide
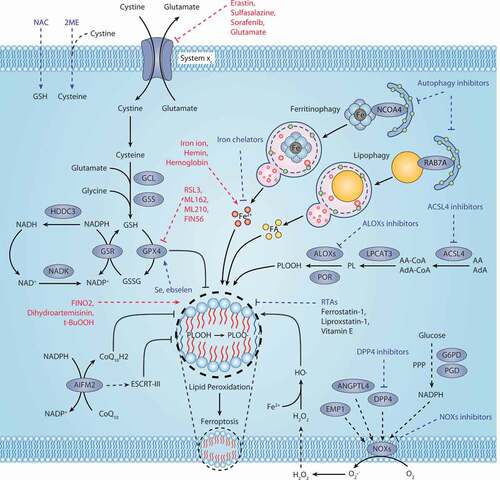
Figure 2. Iron metabolism and ferroptosis. Extracellular iron enters cells, mainly through endosome-mediated internalization of the TF-TFRC complex and receptor-mediated NTBI or heme uptake. Free iron in the cells is stored in ferritin, and iron is released again by ferritinophagy-mediated ferritin degradation. The iron chaperones PCBP1 and PCBP2 may play an important role in cellular iron transmission. SLC40A1 is an iron exporter, cooperating with iron oxidases (e.g., CP, HEPH, and HEPHL1) to transport iron to the extracellular space. Iron, heme, or Fe-S are all incorporated into ROS-generating enzymes (e.g., ALOXs, NOXs, XDH, CYP/CYP450, and ETC complexes) or antioxidant enzymes (e.g., CAT). ACO1 and IREB2 regulate iron homeostasis by controlling the translation of mRNA related to iron metabolism. Abbreviations: ABCB7, ATP binding cassette subfamily B member 7; ACO1, aconitase 1; ALOXs, lipoxygenases; CAT, catalase; CISD1, CDGSH iron sulfur domain 1; CP, ceruloplasmin; CYBRD1, cytochrome B reductase 1; CYP450, cytochrome P450; ETC, electron transport chain; Fe-S, iron-sulfur cluster; FLVCR1, FLVCR heme transporter 1; FLVCR2, FLVCR heme transporter 2; HAMP, hepcidin antimicrobial peptide; HB, hemoglobin; HEPH, hephaestin; HEPHL1, hephaestin like 1; HMOX1, heme oxygenase 1; HP, haptoglobin; HPX, hemopexin; IREB2, iron responsive element binding protein 2; LIP, labile iron pool; LRP1, LDL receptor related protein 1; LTF, lactotransferrin; NCOA4, nuclear receptor coactivator 4; NFS1, NFS1 cysteine desulfurase; NOXs, NADPH oxidases; NTBI, nontransferrin-bound iron; PCBP1, poly(RC) binding protein 1; PCBP2, poly(rC) binding protein 2; POR, cytochrome p450 oxidoreductase; SLC11A2, solute carrier family 11 member 2; SLC25A28, solute carrier family 25 member 28; SLC25A37, solute carrier family 25 member 37; SLC39A8, solute carrier family 39 member 8; SLC39A14, solute carrier family 39 member 14; SLC40A1, solute carrier family 40 member 1; SLC46A1, solute carrier family 46 member 1; STEAP3, STEAP3 metalloreductase; TF, transferrin; TFRC, transferrin receptor; XDH, xanthine dehydrogenase
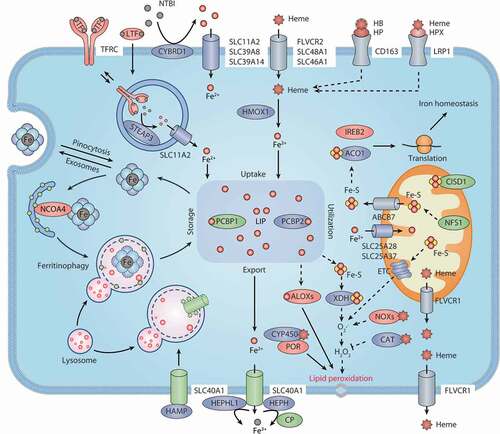
Figure 3. Lipid metabolism and ferroptosis. Both lipid catabolism and anabolic changes affect lipid peroxidation during ferroptosis. De novo lipogenesis is the synthesis of fatty acids from acetyl-CoA produced by the mitochondrial tricarboxylic acid (TCA) cycle. FA is transported into mitochondria for β-oxidation to release acetyl-CoA. Lipid droplets are intracellular sites for neutral lipid storage, and lipophagy induces autophagic degradation of lipid droplets and promotes the release of free FA. Lipolysis is defined as the catabolism of TAG stored in cell lipid droplets, which is regulated by PNPLA2/ATGL, LIPE/HSL, and MGLL. Oxygenases, such as ALOX and POR, catalyze the oxidation of PUFA to activate lipid peroxidation. ACSL4 and LPCAT act as positive regulators of lipid peroxidation by shaping cellular phospholipids. The NOX protein family reduces oxygen to produce free radicals, thereby attacking PUFA-containing lipids. Abbreviations: AA, arachidonic acid; AdA, adrenic acid; ACACs, acetyl-CoA carboxylases; ACLY, ATP citrate lyase; ACSL4, acyl-CoA synthetase long chain family member 4; ACSLs, acyl-CoA synthetase long chain family member proteins; ACSMs, acyl-CoA synthetase medium chain family member proteins; ALOXs, lipoxygenases; CAT, catalase; CoA, coenzyme A; CPT1, carnitine palmitoyltransferase 1; CPT2, carnitine palmitoyltransferase 2; DAG, diacylglycerol; DAGL, diacylglycerol lipase; ETC, electron transport chain; FA, fatty acid; FABPs, fatty acid binding proteins; FASN, fatty acid synthase; GPX4, glutathione peroxidase 4; GPXs, glutathione peroxidases; GSH, glutathione; GSSG, oxidized glutathione; H2O2, hydrogen peroxide; LIPE, lipase E, hormone sensitive type; LPCAT3, lysophosphatidylcholine acyltransferase 3; MAG, monoacylglycerol; MGLL, monoglyceride lipase; NOXs, NADPH oxidases; PE, phosphatidylethanolamine; PL, phospholipid; PNPLA2, patatin like phospholipase domain containing 2; POR, cytochrome p450 oxidoreductase; PRDXs, peroxiredoxins; PUFAs, polyunsaturated fatty acids; RAB7A, RAB7A, member RAS oncogene family; SLC25A20, solute carrier family 25 member 20; SOD1, superoxide dismutase 1; SOD2, superoxide dismutase 2; SOD3, superoxide dismutase 3; TAG, triacylglycerol; TCA cycle, tricarboxylic acid cycle
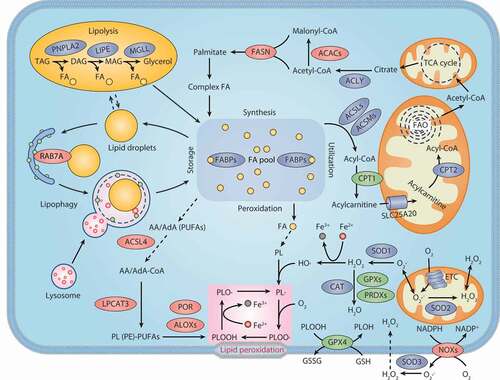
Figure 4. The membrane repair function of ESCRT-III in RCD. ESCRT-III plays a key role in plasma membrane repair in three forms of regulated cell death (RCD), including pyroptosis, necroptosis, and ferroptosis. In pyroptosis and necroptosis, GSDMD and MLKL are transported from the cytoplasm to the cell membrane and form oligomers and large permeability pores, resulting in cell death. In the case of ferroptosis, the accumulation of lethal lipid peroxidation production or the translocation of unknown pore-forming protein in the cell membrane leads to cell death. ESCRT-III machines, including the CHMP family and VPS4, can promote membrane sprouting and shedding of injured plasma membranes. Abbreviations: CASPs, caspases; CHMP2A, charged multivesicular body protein 2A; CHMP3, charged multivesicular body protein 3; CHMP4B, charged multivesicular body protein 4; CHMP5, charged multivesicular body protein 5; CHMP6, charged multivesicular body protein 6; ESCRT-III, endosomal sorting complex required for transport-III; GPX4, glutathione peroxidase 4; GSDMD, gasdermin D; MLKL, mixed lineage kinase domain like pseudokinase; RIPK3, receptor interacting serine/threonine kinase 3; VPS4, vacuolar protein sorting 4
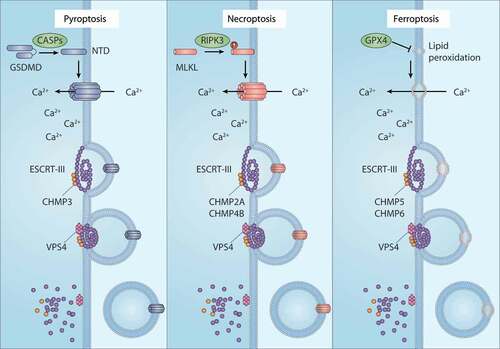
Table 1. Ferroptosis inducers
Table 2. Ferroptosis inhibitors
Table 3. Transcriptional regulators in ferroptosis
Figure 5. Dual role of TP53 in ferroptosis. 1) Pro-ferroptosis role of TP53. TP53 inhibits the expression of SLC7A11 or promotes the expression of SAT1, thus regulating ALOX12- or ALOX15-dependent lipid peroxidation reactions, respectively. 2) Anti-ferroptosis role of TP53. TP53 binds with DDP4 in the nucleus, which limits DPP4 binding to NOX1 to mediate ROS production in the cell membrane. TP53 also promotes the expression of CDKN1A, thereby inducing the production of GSH to inhibit lipid peroxidation. Abbreviations: ALOX12, arachidonate 12-lipoxygenase; ALOX15, arachidonate 15-lipoxygenase; CDKN1A, cyclin dependent kinase inhibitor 1A; DPP4, dipeptidyl peptidase 4; GSH, glutathione; NOX1, NADPH oxidase 1; SAT1, spermidine/spermine N1-acetyltransferase 1; SLC7A11, solute carrier family 7 member 11; TP53, tumor protein P53
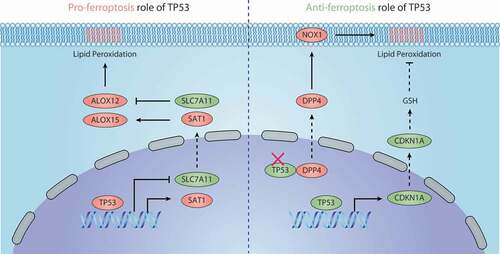
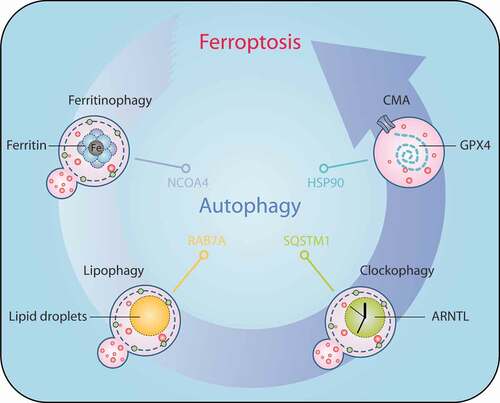
Figure 6. Mechanisms of autophagy-dependent ferroptosis. Several types of selective autophagy, including ferritinophagy, lipophagy, clockophagy, and CMA, promote ferroptotic cell death by inducing the degradation of ferritin, lipid droplets, ARNTL, and GPX4, respectively. Abbreviations: ARNTL, aryl hydrocarbon receptor nuclear translocator like; CMA, chaperone-mediated autophagy; GPX4, glutathione peroxidase 4; HSP90, heat shock protein 90; NCOA4, nuclear receptor coactivator 4; RAB7A, RAB7A, member RAS oncogene family; SQSTM1, sequestosome 1