Figures & data
Figure 1. Increased autophagy in cochlear SGNs after cisplatin treatment in vitro. The middle-turn cochleae and SGNs from P3 C57BL/6 WT mice were cultured and incubated with cisplatin for 48 h. (A) TEM analysis evaluated the autophagy in SGNs. Representative images showed that the ER and Golgi apparatus took on a circular structure, which enveloped the targeted cytoplasmic constituents and formed double membrane vesicles, i.e. autophagosomes (red arrows), that eventually fused with lysosomes and formed autolysosomes (yellow arrows) in which the contents are degraded and recycled by lysosome enzymes, n = 4. Scale bars: 2 μm. (B) Statistical analysis demonstrated that there were significantly more autophagosomes and autolysosomes in the SGNs after cisplatin treatment compared with the controls. (C) Immunostaining demonstrated that the LC3B puncta (LC3B-labeling, red) in the damaged SGNs (TUBB3-labeling, green) were significantly increased after cisplatin administration, n = 6. Scale bars: 5 μm. (D) Quantification of the LC3B fluorescent puncta. (E) The protein expression of LC3B-II in SGNs treated with cisplatin was significantly increased compared to the control group, n = 6. All data are presented as the mean ± SEM, * P < 0.05, ** P < 0.01
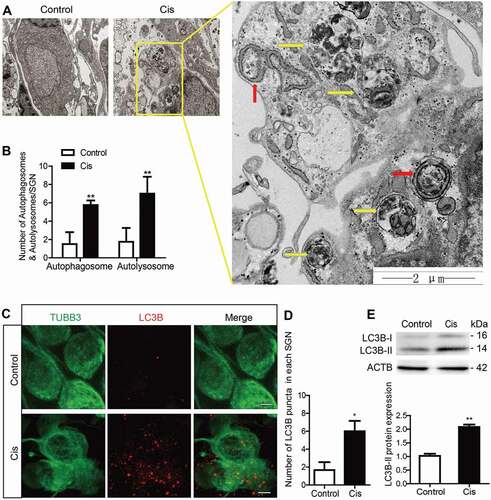
Figure 2. Cisplatin injury activates autophagic flux in the cultured cochlear SGNs. The middle-turn cochlear and SGN explants were cultured in vitro and treated with cisplatin (50 μM) alone or together with Baf (100 nM) for 48 h. (A) Immunostaining showed significantly more LC3B puncta in the Cis + Baf group compared with the Baf-alone group or cisplatin-alone group. Scale bars: 5 μm. (B) Quantification of the LC3B puncta in SGNs. (C) The expression of LC3B-II was significantly increased in the Cis + Baf group compared with Baf alone or cisplatin alone. (D) The protein levels of SQSTM1 were significantly decreased in cisplatin-treated SGNs and increased after Baf treatment compared with control SGNs. (E) Immunostaining showed anti-LC3B (green) and anti-SQSTM1 (red) labeling in SGNs. The colocalization puncta (white arrows) appeared after cisplatin treatment. Scale bars: 5 μm. (F) The cultured cochlear SGNs were incubated with Ad-mRFP-GFP-LC3B for 24 h, and then the medium was replaced with normal medium and the cultured SGNs were further treated with 50 μM cisplatin for 48 h. Immunostaining analysis showed that both the numbers of autophagosomes (yellow puncta, yellow arrows) and autolysosomes (red puncta, red arrows) were significantly increased in cisplatin-treated SGNs compared with the control group, n = 6 for each subtype. Scale bars: 5 μm. All data are presented as the mean ± SEM, * P < 0.05, ** P < 0.01, *** P < 0.001
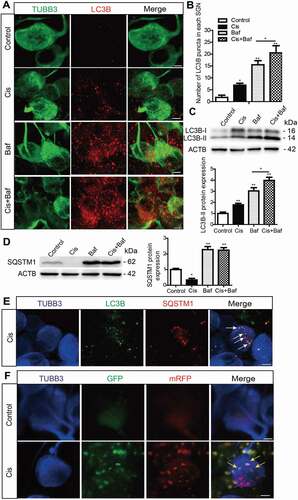
Figure 3. Autophagy inhibits apoptosis and promotes the survival of SGNs after cisplatin injury in vitro. The cultured cochlear SGNs were pretreated with RAP (0.1 μM) or 3-MA (5 mM) for 6 h and then co-treated with 50 μM cisplatin for 48 h. (A and B) Immunostaining and quantification showed that the number of LC3B puncta (red) was significantly increased in the Cis + RAP group and decreased in the Cis + 3-MA group compared with cisplatin treatment alone. Scale bars: 5 μm. (C) The LC3B-II protein expression was significantly increased in the Cis + RAP group and decreased in the Cis + 3-MA group compared with cisplatin treatment alone. (D and E) SGN counting showed that RAP co-treatment promoted SGN survival compared with cisplatin exposure alone, while 3-MA accelerated SGN loss after cisplatin injury, and the number of SGNs was not significantly changed when cells were treated with 0.1 μM RAP or 5 mM 3-MA alone without cisplatin. Scale bars: 25 μm. (F and G) Fewer cleaved-CASP3-positive SGNs and lower protein levels of cleaved-CASP3were found in the Cis + RAP group, whereas the 3-MA co-treated SGNs had significantly more cleaved-CASP3-positive SGNs and higher cleaved-CASP3 protein expression compared with the cisplatin-only group. Scale bars: 25 μm. (H) The mRNA expression of Casp3, Casp8, Casp9, and Bax was significantly reduced in the Cis + RAP group and was significantly increased in the Cis + 3-MA SGNs compared to the cisplatin-only group, while the lower mRNA expression of the antiapoptotic gene Bcl-2 was enhanced in the Cis + RAP group. C-CASP3, cleaved-CASP3. n = 6 for each subgroup. All data are presented as the mean ± SEM, * P < 0.05, ** P < 0.01, *** P < 0.001
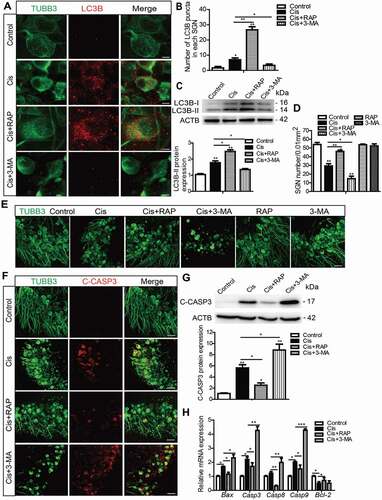
Figure 4. Autophagy inhibits apoptosis, promotes the survival of SGNs, and protects hearing function after cisplatin-induced damage in vivo. WT mice were injected i.p. with 3 mg/kg cisplatin daily for 7 days starting at P30 alone or co-treated with 1 mg/kg RAP i.p. every other day from P14 until P36, or with 15 mg/kg 3-MA i.p. every day from P14 until P36. (A) The ABR thresholds of all frequencies were increased in the cisplatin-treated group compared with the control group, and the shifts of thresholds were significantly lower in the Cis + RAP group, while they were much higher in the Cis + 3-MA group, compared to those in the cisplatin-only mice. (B and C) CAP threshold elevation (B) and amplitude reduction measured at 90 dB SPL (C) were observed across all tested frequencies. Co-treatment with RAP significantly decreased the CAP threshold and increased amplitudes compared with the cisplatin-only treatment in mice, whereas 3-MA administration had the opposite effect. (D and F) The LC3B puncta (D, red) and LC3B-II protein expression (F) in SGNs were increased after cisplatin administration, and RAP co-treatment further upregulated these, while 3-MA further downregulated them compared with the cisplatin-only group. Scale bars: 5 μm. (E and G) There were fewer cleaved-CASP3-positive SGNs and the protein level of cleaved-CASP3 was reduced in the Cis + RAP group compared with the cisplatin-only controls, while they were both increased in the Cis + 3-MA group. Scale bars: 12.5 μm. (H and I) Pretreatment with RAP protected SGN survival compared with the cisplatin-only group, while 3-MA accelerated SGN loss after cisplatin injury, and RAP or 3-MA treatment alone did not cause any significant differences in SGN numbers compared to the control group in the absence of cisplatin damage. Scale bars: 25 μm. n = 6 for each group. All data are presented as the mean ± SEM, * P < 0.05, ** P < 0.01
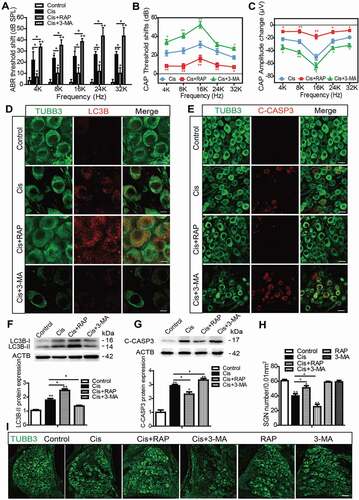
Figure 5. Autophagy attenuates cisplatin-induced oxidative stress in SGNs. The cultured cochlear SGNs were pretreated with RAP (0.1 μM) or 3-MA (5 mM) for 6 h and then co-treated with 50 μM cisplatin for 48 h. (A and B) Compared with the cisplatin group, the immunostaining signals (A) and the protein levels of 4-HNE (B) in SGNs were significantly reduced after RAP cotreatment, while they were further increased in the Cis + 3-MA group. Scale bars: 12.5 μm. (C) RAP co-treatment significantly upregulated the mRNA expressions of Sod1, Gsr, Glrx, and Cat, while 3-MA co-treatment significantly downregulated the mRNA expressions of Sod1, Gsr, Glrx, Tmx3, and Nqo1 compared with the cisplatin-only group. (D) There were no statistically significant changes of mitochondrial protein levels (TOMM20 and COX4I1/COX IV) in our experiments. n = 6 for each group. All data are presented as the mean ± SEM, * P < 0.05, ** P < 0.01
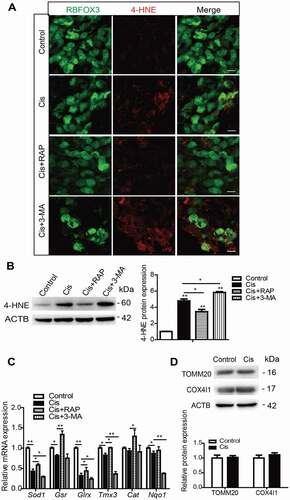
Figure 6. Antioxidant treatment successfully rescued the increased SGN loss induced by autophagy inhibition after cisplatin injury. The cultured middle turn cochleae were treated with cisplatin (50 μM) alone, cotreated with NAC (2 mM), or pretreated with 3-MA (5 mM) for 6 h then cotreated with NAC for 48 h. (A and B) Immunofluorescence signals (A) and the protein expression levels (B) of 4-HNE were lower in the Cis + NAC group compared to the cisplatin-only group, and they were also reduced in the Cis + 3-MA + NAC group compared to the Cis + 3-MA group. Scale bars: 12.5 μm. (C-E) The NAC treatment significantly increased the number of surviving SGNs (C) and decreased the expression of cleaved-CASP3 (D and E) in the Cis + NAC group compared to the cisplatin-only group, as well as in Cis + 3-MA + NAC group versus the Cis + 3-MA group. Scale bars: 25 μm. n = 6 for each subgroup. All data are presented as the mean ± SEM, * P < 0.05, ** P < 0.01
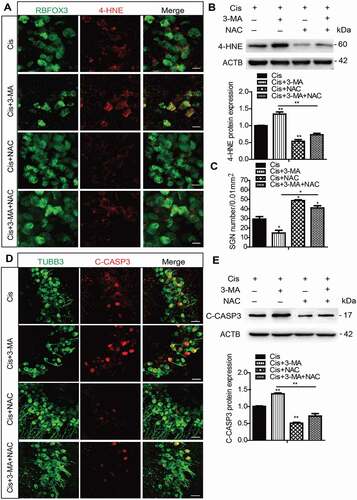
Figure 7. PRDX1 is activated and regulates the autophagy activity in SGNs after cisplatin injury. The middle-turn cochleae and SGNs from P3 C57BL/6 WT mice or prdx1−/− mice were cultured and incubated with cisplatin for 48 h. (A and B) Immunostaining and western blot revealed that the expression of PRDX1 was significantly increased in SGNs after cisplatin administration. Scale bars: 12.5 μm. (C and D) The number of surviving SGNs was significantly reduced in prdx1−/− mice after cisplatin administration, while there was no difference in the SGN numbers in prdx1−/− mice compared to the WT control mice without cisplatin damage. Scale bars: 25 μm. (E-G) The puncta numbers of LC3B (E and F) and the protein expression of LC3B-II (G) were downregulated significantly in the Cis + prdx1−/− group compared with the cisplatin-only group. Scale bars: 5 μm. n = 6 for each subgroup. All data are presented as the mean ± SEM, * P < 0.05, ** P < 0.01
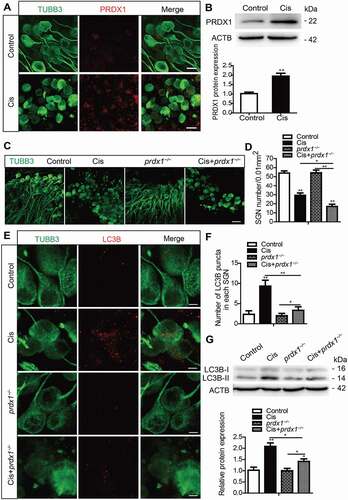
Figure 8. Ebselen and overexpression of PRDX1 protect against cisplatin-induced ROS accumulation and apoptosis and reduce SGN loss by activating autophagy in SGNs. The cultured cochleae from WT mice were treated with cisplatin (50 μM) alone, cotreated with ebselen (30 μM, pretreated for 1 h), cotreated with Anc80-Prdx1 (4 × 1010 GC/mL, preincubation for 24 h), or cotreated with 3-MA (5 mM, pretreated for 6 h) for 48 h. (A) RT-PCR results showed that the mRNA expression of genes associated with autophagic flux, including Atg5, Becn1, Lc3b, Ctsb, and Lamp1, was upregulated in SGNs induced by cisplatin, and the overexpression of PRDX1 by ebselen or Anc80-Prdx1 further enhanced the above gene transcriptions compared to the cisplatin-only group. (B) Ebselen and Anc80-Prdx1 both significantly increased the protein expression of LC3B-II compared to the cisplatin-only group, whereas the co-treatment with 3-MA effectively prevented the upregulation of LC3B-II expression induced by ebselen or Anc80-Prdx1. (C-G) The expressions of 4-HNE (C and D) and cleaved-CASP3 (F and G) were significantly decreased, and the number of surviving SGNs (E) was significantly increased in the Cis + ebselen group and in the Cis + Anc80- Prdx1 group compared to the cisplatin-only group, while co-treatment with 3-MA significantly reduced the effects of both ebselen and Anc80-Prdx1 on SGN ROS production, cell survival, and apoptosis. Scale bars: 12.5 μm in (C). Scale bars: 25 μm in (F). n = 6 for each subgroup. All data are presented as the mean ± SEM, * P < 0.05, ** P < 0.01
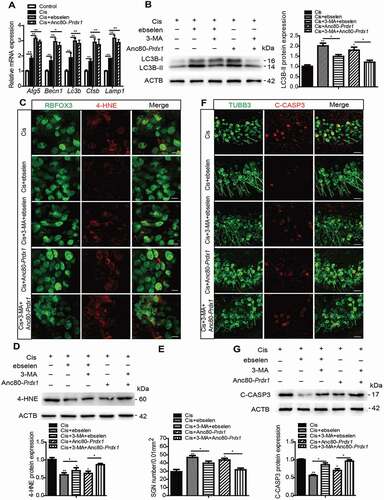
Figure 9. Ebselen improves hearing function, promotes SGN survival, and inhibits SGN apoptosis after cisplatin-induced damage in vivo by activating autophagy. WT mice were injected i.p. with 3 mg/kg cisplatin alone daily for 7 days starting at P30 or with 15 mg/kg 3-MA i.p. every day from P14 until P36 or were co-injected i.p. with 15 mg/kg ebselen daily from P30 for 7 days. (A and B) The CAP threshold shifts (A) and amplitude changes (B) of all frequencies were both lower in the Cis + ebselen group than in the cisplatin-only mice, while in the Cis + ebselen + 3-MA group the CAP threshold shifts and amplitude changes were significantly increased compared with the Cis + ebselen group. (C) Ebselen administration significantly increased the protein expression of LC3B-II in SGNs compared to the cisplatin-only group, while 3-MA treatment inhibited the upregulation of LC3B in the Cis + ebselen + 3-MA group compared to the Cis + ebselen group. (D and E) The mean density of SGNs was increased after co-treatment with ebselen compared to the cisplatin-only mice, but decreased in the Cis + ebselen + 3-MA group compared with the Cis + ebselen group, and the number of SGNs was not significantly changed after mice were treated with ebselen or 3-MA alone without cisplatin injury. Scale bars: 25 μm. (F and G) The expression of cleaved-CASP3 was decreased in mice after co-treatment with ebselen compared to the cisplatin-only mice, whereas it decreased significantly in the Cis + ebselen + 3-MA group compared with the Cis + ebselen group, n = 6 for each group. Scale bars: 12.5 μm. All data are presented as the mean ± SEM, *P < 0.05, **P < 0.01
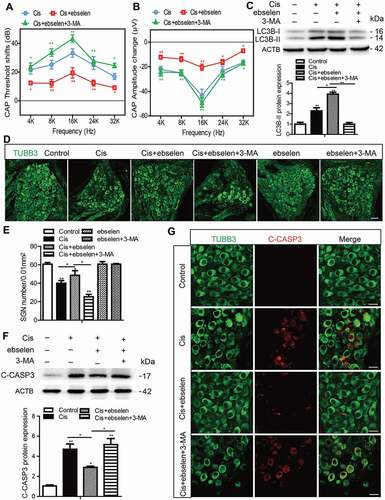
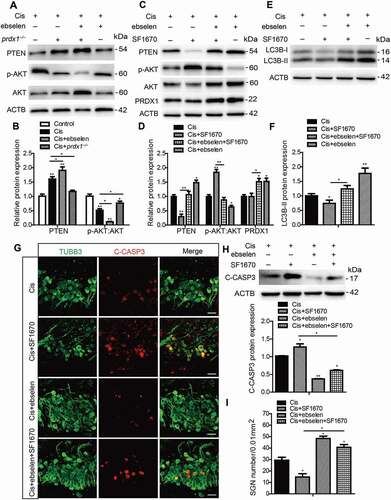
Figure 10. PRDX1 regulates the PTEN-AKT signaling pathway to activate autophagy in SGNs after cisplatin treatment. (A and B) The cultured cochleae and SGNs from WT mice or prdx1−/− mice were incubated with cisplatin (50 μM) for 48 h or cotreated with ebselen (30 μM, pretreated for 1 h) and cisplatin (50 μM) for 48 h. The protein expression of PTEN was significantly increased and the ratio of p-AKT:AKT was significantly decreased in cultured SGNs after cisplatin injury. Treatment with ebselen caused increased expression of PTEN and a lower ratio of p-AKT:AKT in SGNs compared to the cisplatin-only group, while the lack of PRDX1 in SGNs from prdx1−/− mice led to an opposite changing pattern. (C and D) The cultured cochleae and SGNs from WT mice were incubated with cisplatin (50 μM) alone, cotreated with ebselen (30 μM, pretreated for 1 h), or cotreated with SF1670 (15 μM) for 48 h. SF1670 effectively inhibited the expression of PTEN and enhanced the phosphorylation of AKT in SGNs after cisplatin treatment, and co-treatment with ebselen reversed the effect of SF1670 as the expression of PTEN was increased and the ratio of p-AKT:AKT was decreased in the Cis + ebselen + SF1670 group compared to the Cis + SF1670 group. In contrast, treatment with SF1670 did not change the expression of PRDX1 in SGNs after cisplatin incubation. (E-I) The inhibition of PTEN by SF1670 significantly decreased the expression of LC3B-II (E and F), increased cleaved-CASP3 expression (G and H), and reduced the number of surviving SGNs (I) compared to the cisplatin-only group, whereas co-treatment with ebselen rescued the above effect of SF1670 in SGNs. Scale bars: 25 μm. n = 6 for each group. All data are presented as the mean ± SEM, * P < 0.05, ** P < 0.01