Figures & data
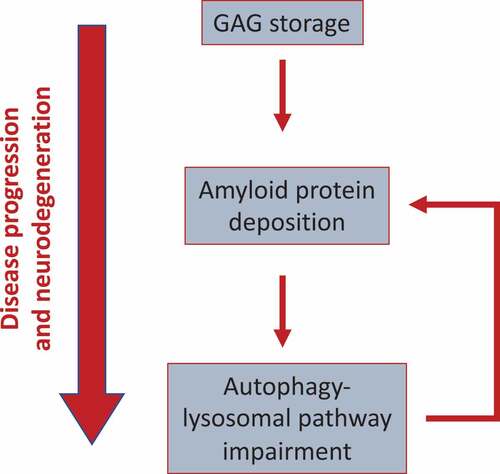
Figure 1. Model showing the functional link between GAGs, amyloid aggregation, and ALP dysfunction in MPS-III. GAG storage initially drives protein aggregation (likely, because of the capability of GAGs to provide a scaffold promoting amyloid aggregation). Perikaryal protein aggregation, in turns, triggers the block of the autophagy flux observed in MPS-III, likely by affecting lysosomal dynamics and trafficking. Then, because the ALP itself plays a key role in the clearance of protein aggregates, this may generate a vicious cycle, which boosts amyloid buildup and toxicity. This cascade of events drives disease progression and neurodegeneration