Figures & data
Figure 1. GTPBP4 promotes FMDV replication in cells. PK-15 cells transfected with 150 nM of GTPBP4 siRNA or NC siRNA were infected with FMDV (MOI 0.1) (A) PK-15 cells transfected with increasing flag-GTPBP4 expression plasmid (0, 1, and 2 μg) were infected with FMDV (MOI 0.1) (B) HT-29 cells transfected with 150 nM of GTPBP4 siRNA or NC siRNA were infected with EV71 (MOI 1) (C) the viral titers in the supernatant were determined by TCID50 assay.
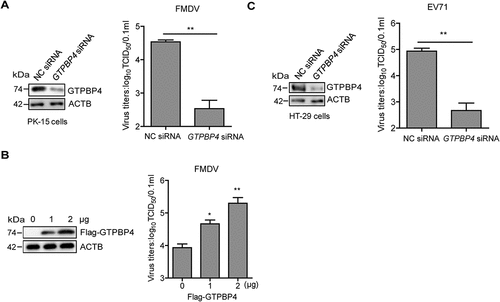
Figure 2. GTPBP4-deficient mice are more resistant to FMDV infection. (A) the expression of GTPBP4 in the carcasses without the head, tail, limbs, and viscera of WT and Gtpbp4± mice was detected by western blotting. (B-E) the three-day-old WT and Gtpbp4± mice were subcutaneously inoculated with FMDV (108 TCID50). FMDV titers in the mice carcasses without the head, tail, limbs, and viscera were determined by TCID50 assay (B). The mortality of WT and Gtpbp4± mice (n = 10) was determined (C). H&E staining was performed for histological examination of the lung (D) and liver (E) of mice. A black arrowhead indicates inflammatory cells in the liver. (F-G) the three-day-old WT and Gtpbp4± mice were subcutaneously inoculated with EV71 (108 TCID50). The viral titers in the mice carcasses without the head, tail, limbs, and viscera were determined at 2 dpi by TCID50 assay. The mortality of mice (n = 5) was determined.
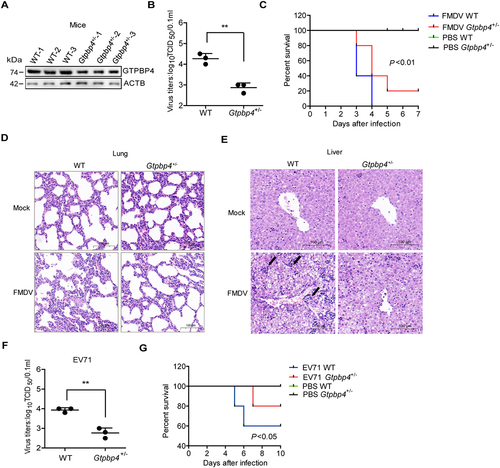
Figure 3. GTPBP4 involves in FMDV-induced type I interferon production. (A) PK-15 cells transfected with 150 nM of GTPBP4 siRNA or NC siRNA were infected with FMDV (MOI 0.1). The mRNA expression of IFNB, ISG15, and IFIT2 was measured by qPCR. The level of IFNB protein in the supernatant was detected by ELISA kit (B). (C-D) the three-day-old WT and Gtpbp4± mice (n = 4) were subcutaneously inoculated with FMDV (108 TCID50) or EV71 (108 TCID50). The mRNA expression of Ifnb, Isg15, and Ifit2 in FMDV-infected mice was measured by qPCR (C). The expression of ifnb protein in the mouse serum was detected by ELISA kit (D).
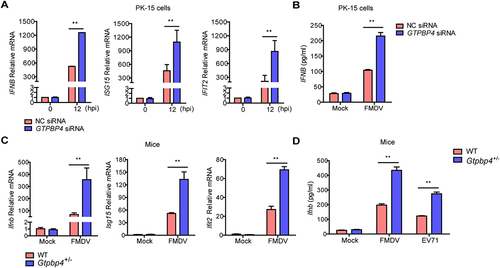
Figure 4. FMDV infection promotes the expression of GTPBP4. (A) PK-15 cells were mock-infected and infected with FMDV (MOI 0.1) for 0, 6, and 12 h. The expression of GTPBP4 protein and mRNA was detected by western blotting and qPCR, respectively. (B) the three-day-old WT mice were subcutaneously inoculated with or without FMDV (108 TCID50) for 2 d. The expression of Gtpbp4 protein and mRNA in the mice carcasses without the head, tail, limbs, and viscera was detected by western blotting and qPCR, respectively. (C) HT-29 cells were mock-infected or infected with EV71 (MOI 1) for 0, 12, and 24 h. The abundance of GTPBP4 protein was determined by western blotting.
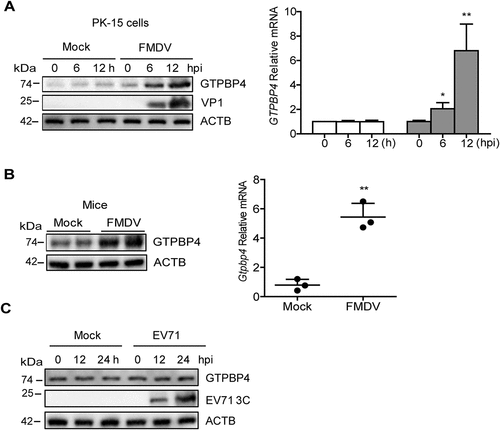
Figure 5. FMDV VP1 was responsible for the increase of GTPBP4. (A) PK-15 cells were transfected with 2 μg of plasmids expressing flag-VP1 proteins. The expression of GTPBP4 protein and mRNA was detected by western blotting and qPCR, respectively. (B) schematic representation showing a series of flag-tagged truncated VP1 mutants. (C-E) PK-15 cells were transfected with 2 μg of empty vector, flag-VP1- or the indicated flag-VP1-mutants-expressing plasmids. At 24 hpt, the expression of GTPBP4 protein was determined by western blotting. The effect of Flag-VP1Q209A on GTPBP4 mRNA expression was detected by qPCR. (F) PK-15 cells transfected with empty vector, flag-VP1, or Flag-VP1Q209A expression plasmid were infected with SeV for 12 h. Chromatin was immunoprecipitated with an anti-IRF3 antibody. The impact of VP1 on IRF3 binding onto IFNB promoter was analyzed by quantitative ChIP assay (left). The abundance of the immunoprecipitated DNA was normalized to the input DNA levels. The expression of IFNB protein in the supernatant was detected by ELISA kit (right). (G) PK-15 cells were mock-infected and infected with WT FMDV or FMDV-VP1Q209A for 8 h. The expression of GTPBP4 protein was detected by western blotting. The level of IFNB protein in the supernatant was detected by ELISA kit. (H-J) the three-day-old WT mice were subcutaneously inoculated with WT FMDV (108 TCID50) or FMDV-VP1Q209A (108 TCID50). The expression of Ifnb protein in the mice serum was detected by ELISA kit (H). The mortality of WT FMDV- and FMDV-VP1Q209A-infected mice (n = 10) was determined (I). H&E staining was performed for histological examination of the lung in mice (J).
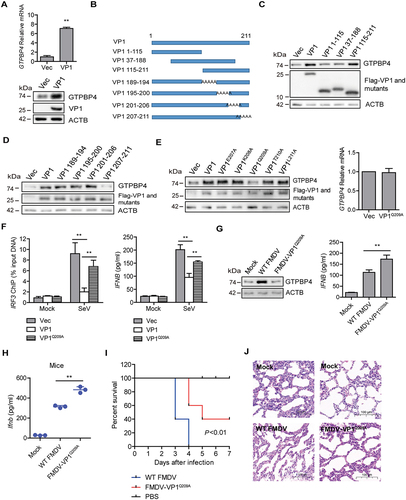
Figure 6. FMDV VP1 enhances GTPBP4 expression by degrading YTHDF2. (A) the protein and mRNA levels of GTPBP4 in the WT and YTHDF2−/− PK-15 cells were detected by western blotting and qPCR, respectively. (B) total RNA was isolated from PK-15 cells. The RNA was immunoprecipitated with m6A-specific antibody. The immunoprecipitated RNA was reverse-transcribed to cDNA. The methylation of GTPBP4 was determined by paired-end sequencing. s: sample. (C) PK-15 cells transfected with 5 μg of flag-YTHDF2 expression plasmid were collected and immunoprecipitated using anti-IgG or anti-flag antibody (left). Total RNA was immunoprecipitated with m6A-specific antibody (right). The immunoprecipitated RNA was reverse-transcribed to cDNA. The levels of methylated GTPBP4 mRNA were detected by qPCR. (D) PK-15 cells were transfected with 2 μg of flag-YTHDF2 or flag-YTHDF2-ΔYTH expression plasmid for 24 h. The protein expression of GTPBP4 and YTHDF2 was detected by western blotting. (E-F) PK-15 cells were mock-infected or infected with WT FMDV or FMDV-VP1Q209A. The expression of YTHDF2 protein was detected by western blotting. (G) PK-15 cells were transfected with 2 μg of flag-VP1 or Flag-VP1Q209A expression plasmid for 24 h. The protein expression of YTHDF2 was detected by western blotting. (H) YTHDF2−/− cells were transfected with 2 μg of empty vector or flag-VP1 expression plasmid for 24 h. The expression of GTPBP4 and YTHDF2 was detected by western blotting. (I-J) PK-15 cells were mock-infected or infected with FMDV (MOI 0.1) for 6 h. The cell lysates were immunoprecipitated with anti-YTHDF2 or anti-VP1 antibodies. The antibody-antigen complexes were analyzed by the indicated antibodies (I). The intracellular localization of YTHDF2 and VP1 was detected by IFA using anti-YTHDF2 and anti-VP1 antibodies (J).
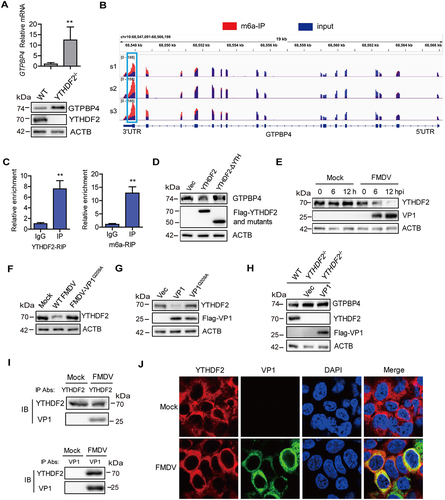
Figure 7. FMDV VP1 promotes YTHDF2 degradation through an AKT-MTOR-dependent autophagy pathway. (A) PK-15 cells were infected with FMDV (MOI 0.1). At 1 hpi, the cells were maintained in the fresh medium in the presence or absence of MG132 (20 μM), 3-MA (1 mM), or CQ (100 μM). At 12 hpi, the abundance of YTHDF2 was determined by western blotting. (B) PK-15 cells were transfected with 2 μg of flag-VP1-expressing plasmid. At 6 hpt, the cells were maintained in the fresh medium in the presence or absence of MG132 (20 μM), 3-MA (1 mM), or CQ (100 μM) for 18 h. Expression of YTHDF2 protein was determined by western blotting. (C) PK-15 cells were incubated with 3-MA (1 mM) or CQ (100 μM) for 18 h. Expression of LC3-I and LC3-II protein was determined by western blotting. (D) PK-15 cells were transfected with 2 μg of GFP-LC3 and flag-VP1-expressing plasmids for 24 h. The autophagosomes were detected using a confocal laser scanning microscope. (E) PK-15 cells were transfected with 2 μg of empty vector or flag-VP1-expressing plasmids for 24 h. The samples were analyzed by transmission electron microscopy to show autophagosomes. (F-H) PK-15 cells were transfected with 2 μg of empty vector, flag-VP1-, or Flag-VP1Q209A-expressing plasmids for 24 h (F and H). PK-15 cells were incubated with the purified flag-VP1 (150 μg/mL) (G). The cells were collected and subjected to western blotting analysis. (I) PK-15 cells were transfected with 2 μg of empty vector or flag-VP1-expressing plasmids for 24 h and maintained in the presence or absence of SC79 (10 μM) for 12 h. The cells were collected and subjected to western blotting analysis. (J) ATG7−/− cells were transfected with 2 μg of empty vector or flag-VP1-expressing plasmids for 24 h. The expression of GTPBP4, YTHDF2, and LC3 was detected by western blotting.
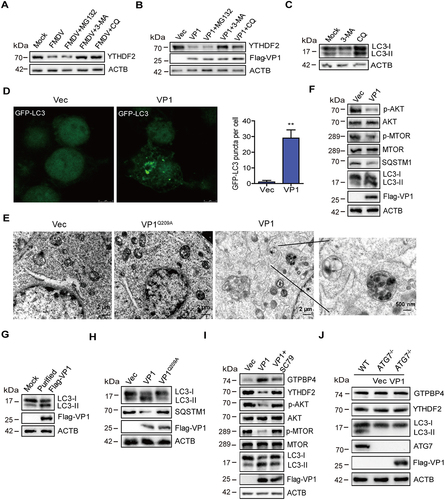
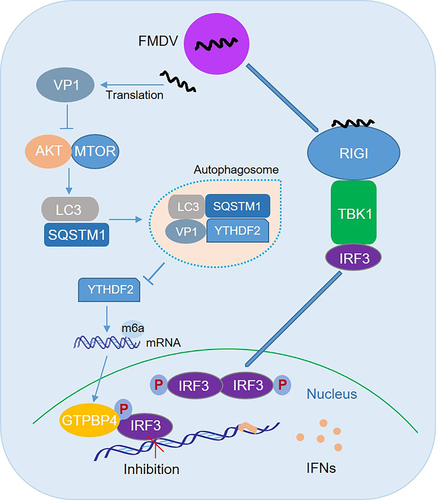
Figure 8. Schematic representation of the model of GTPBP4 and YTHDF2 in innate immune response and autophagy. In this model, FMDV structural protein VP1 interacts with and degrades YTHDF2 in an AKT-MTOR-dependent autophagy pathway, resulting in an increase in GTPBP4 mRNA and protein levels. Increased GTPBP4 inhibits IRF3 binding to the IFNB promoter, suppressing FMDV-induced type I interferon production and promoting viral replication.