Figures & data
Table 1. Laboratory data on admission.
Figure 1. Fomepizole concentration in plasma, post-filter and dialysate in patient 2. CVVHD: Continuous veno-venous hemodialysis; IHD: Intermittent hemodialysis.
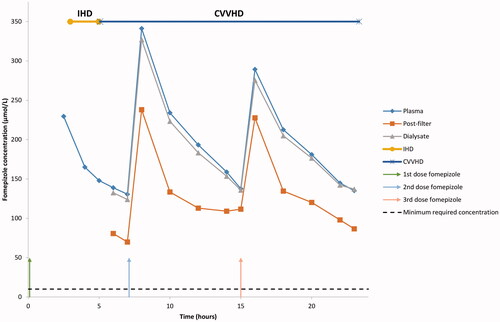
Figure 2. Fomepizole concentration in plasma in patient 1. CVVHD: continuous veno-venous hemodialysis; IHD: intermittent hemodialysis.
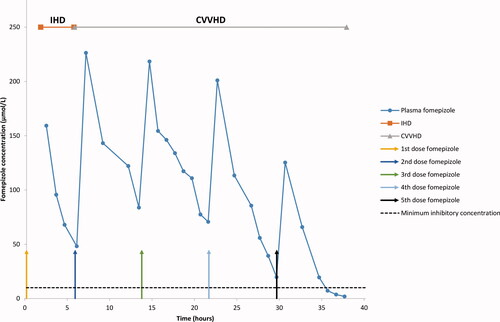
Table 2. Elimination kinetics of fomepizole during continuous renal replacement therapy (CRRT).
Table 3. Dialysis modality, settings and clearance for fomepizole during continuous renal replacement therapy (CRRT).
