Figures & data
Figure 1. (a) Elution profile on sephacryl 100-HR column of midgut crude extract of D. Maroccanus; (b) Chromatogram obtained from ionic exchange DEAE cellulose chromatography of D. Maroccanus midgut extract. (♦) protein absorbance at 280 nm; and (dashed line) NaCl gradient; (c) Analysis of purified α-amylase by SDS-PAGE. Lane a: Crude extract of midgut extract of D. maroccanus, Lane b: purified α-amylase Lane c: Molecular weight of markers: lysozyme (14.4 kDa); lactoglobulin (18.4 kDa); restriction endonuclease bsp 981 (25 kDa); lactate dehydrogenase (35.5 kDa); ovalbumin (45 kDa); bovine serum albumin (66 kDa); α-galactosidase (116 kDa).
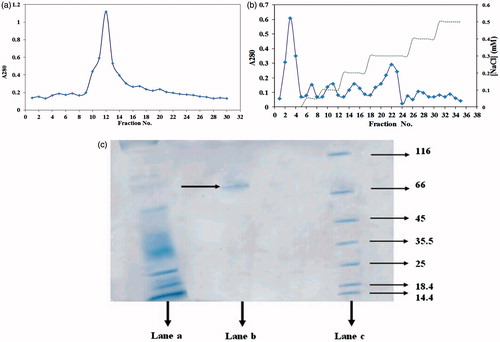
Table 1. Purification of α-amylase from digestive system of D. maroccanus.
Figure 2. (a) The effect of pHs on the mean relative activities of α-amylase purified from the digestive system of D. maroccanus; (b) The effect of temperatures on the mean relative activities of α-amylase purified from the digestive system of D. maroccanus.
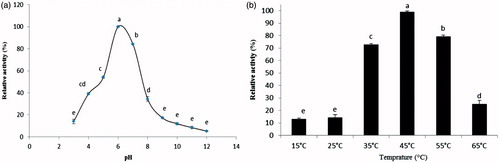
Table 2. Effect of some organic and inorganic compounds on α-amylase activity (iso-enzyme 3) of D. maroccanus..
Figure 3. (a) Elution profile for inhibitor extracted from seeds of P. vulgaris using sephacryl 100-HR column chromatography; (b) Elution profile for inhibitor extracted from seeds of P. vulgaris using ionic exchange DEAE cellulose as bed, equilibrated with 20 mM Tris-HCl buffer. (♦) protein absorbance at 280 nm; and (dashed line) NaCl gradient; (c) SDS–PAGE of proteins at different process of α-amylase inhibitor purification. Lane a: purified P. vulgaris amylase inhibitor (Fraction 17), lane b: crude plant extract, Lane c: purified P. vulgaris amylase inhibitor (Fraction 24), lane d: Molecular weight markers: lysozyme (14.4 kDa); lactoglobulin (18.4 kDa); restriction endonuclease bsp 981 (25 kDa); lactate dehydrogenase (35.5 kDa); ovalbumin (45 kDa); bovine serum albumin (66 KDa); α-galactosidase (116 kDa); (d) molecular weights-relative mobility of proteins for determination of purified α-amylase inhibitor.
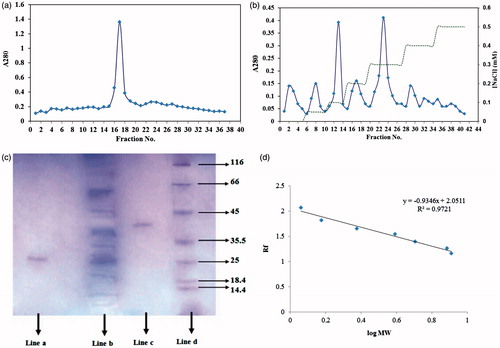
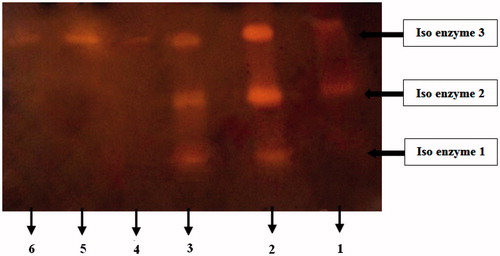
Figure 4. Zymogram of α-amylases extracted from the digestive system of D. maroccanus. Enzyme extract was pre-incubated with P. vulgaris inhibitor (AI1) for 30 min and then gel was run at 4 °C. Lane numbers are as follows: (1) Crude enzyme extract treated with AI1, (2) Crude enzyme extract with no inhibitor, (3) Crude enzyme extract treated with AI2, (4) Purified α- amylase incubated with AI1, (5) Control (Purified α- amylase with no inhibitor), (6) Purified α- amylase incubated with AI2.