Figures & data
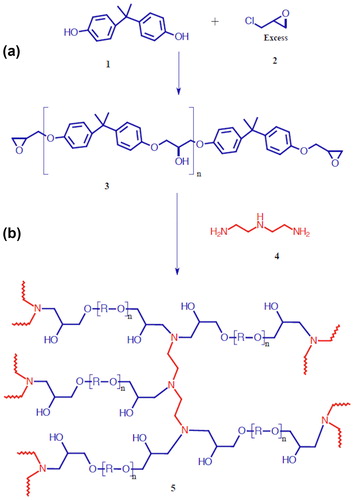
Scheme 1. Formation of a crosslinked epoxy network. (a) Synthesis of DGEBA from bisphenol-A and epichlorohydrin. (b) Polymerization using an amine as a crosslinker.
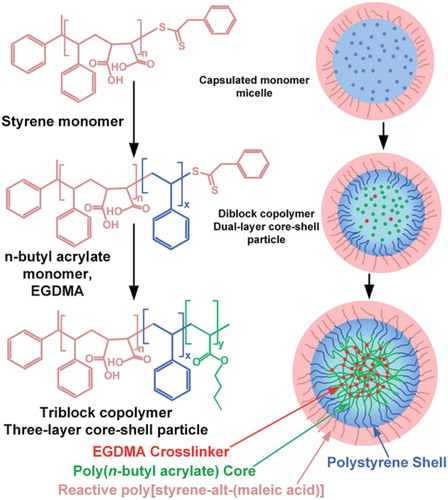
Scheme 2. An illustration of the synthesis of core-shell nanoparticles and the micellar self-assembly of reactive block copolymers. Adapted from Ref. Citation55, with permission from the Royal Society of Chemistry.
Scheme 3. An illustration of the formation of sphere-like nanostructures using reactive block copolymers. Adapted with permission from Ref. Citation58, Copyright (2014) American Chemical Society.
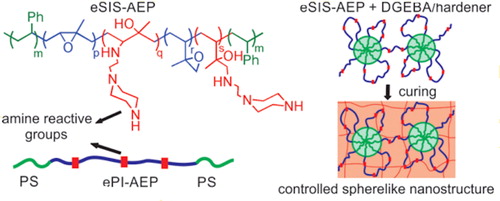
Figure 2. An illustration of network rearrangement with covalent adaptable networks. (a) Prior to bond exchange reaction, where an active unit attaches to an existing bond. (b) Formation of a metastable tertiary structure, leading to one unit being kicked off. (c) Post bond exchange, where a new bond and active unit is formed. Adapted from Ref. Citation73, with permission from the Royal Society of Chemistry.
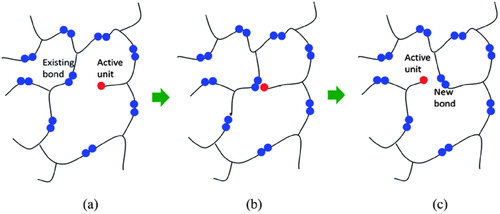
Figure 1. Structures of common ionic liquids used with epoxy resin. (a) A polymeric ionic liquid. (b,c) Phosphonium based ionic liquids.
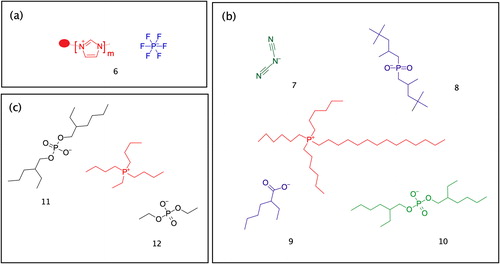
Figure 3. A qualitative assessment of shape memory and permanent shape changing for epoxy vitrimer cross-linked with (CA) + (SA). Adapted from Ref. Citation34, with permission from the Royal Society of Chemistry.
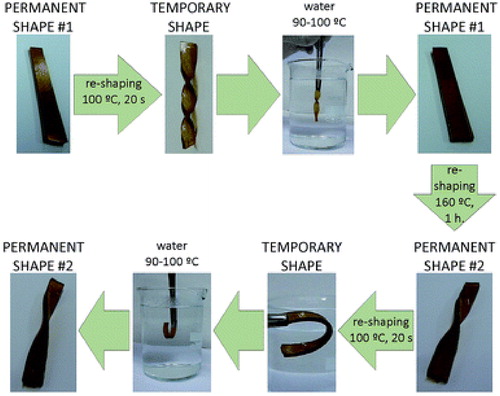
Figure 4. Inducement of flexibility in epoxy thermoset using ionic liquids. Adapted from Ref. Citation80, with permission from The Royal Society of Chemistry. (a) Reversible bending and twisting on application of heat and force with retention of the original shape on cooling. (b) Bending and twisting at room temperature after application of small force with retention of new shape even after removal of force. (c) Bending and twisting after application of small force with retention of original shape after removal of force. (d) Elastomeric thermoset before and after 280% elongation. (e) Thermoset transparency (1) hard thermoset at 10% IL (2) plastic thermoset at 30% IL (3) elastomeric thermoset at 50% IL.
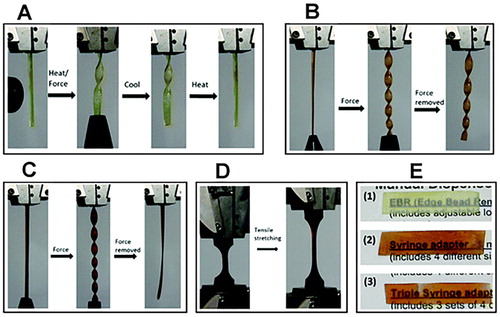
Figure 5. An illustration of the photo welding approach by Yang et al. Adopted from Ref. Citation94, with permission from The Royal Society of Chemistry. (a) Joining non-CNT vitrimer with CNT-vitrimer. (b) Joining two pieces of non-CNT vitrimer using CNT vitrimer as an adhesive. (c) Joining normal epoxy with CNT-vitrimer. (d) Joining thermoplastic PE with CNT-vitrimer.
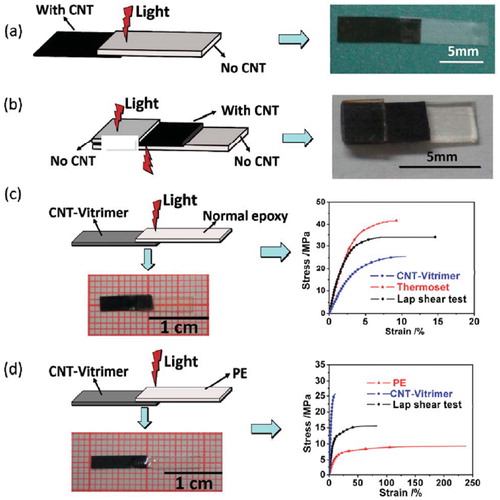
Figure 6. An illustration of bond breaking and reforming during trans-esterification reaction. Adapted with permission form Ref. Citation33, Copyright (2011), American Association for the Advancement of Science. (a) Schematic view of a network during bond exchange process preserving network integrity. (b) Exchange processes via transesterification in hydroxyl-ester networks.
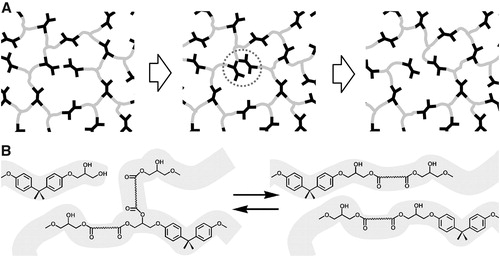
Figure 7. Bond-forming and breaking with disulfide bridges. Adapted with permission from Ref. Citation115, Copyright 2015, Elsevier Ltd. (a) Employed by Luzuriaga et al. in polymer recycling. (b) Employed by Takahashi et al. in polymer recycling.
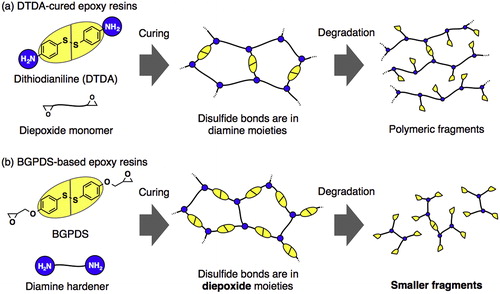
Figure 8. Overview of elastic modulus vs healing temperature of intrinsically self-healing polymeric matrices. Adapted with permission from Ref. Citation116, Copyright 2017, Elsevier Ltd.
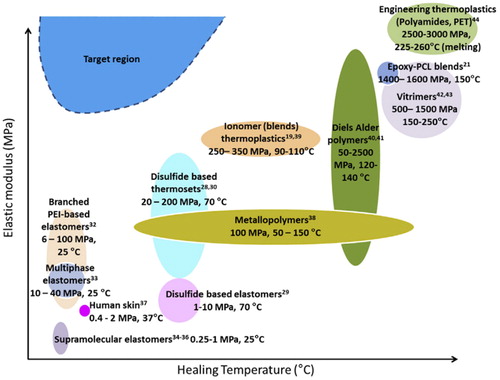
Figure 9. An illustration of self-healing studied under profilometry surface topography. (a) For a neat epoxy. (b) Epoxy with ionic liquid [OMIM] [BF4], showing continuous reduction on wear groves with time. Adapted by permission from Springer Nature: Tribol. Lett., Ref. Citation83.
![Figure 9. An illustration of self-healing studied under profilometry surface topography. (a) For a neat epoxy. (b) Epoxy with ionic liquid [OMIM] [BF4], showing continuous reduction on wear groves with time. Adapted by permission from Springer Nature: Tribol. Lett., Ref. Citation83.](/cms/asset/cb26b979-a1c7-47fe-a5a6-15f4742c2b98/lmsc_a_1650063_f0009_c.jpg)
Figure 11. Schematic of the CNT network forming process. (a) Involving a randomly aligned CNT. (b) With electrical polarization and alignment. (c) interaction between aligned CNT and (d) CNT move closer and form network of aligned bundles. (e) Electrical conductivities of pure epoxy and the nanocomposite with aligned MWCNTs in parallel (//) and perpendicular (⊥) direction with the applied electric field. Adapted with permission from Ref. Citation151, Copyright 2014, Elsevier Ltd.
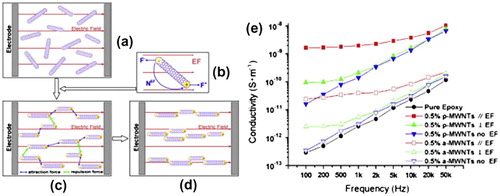
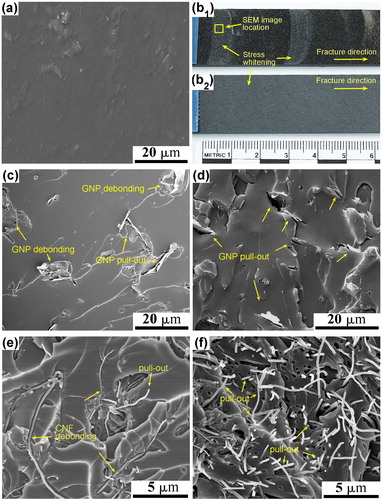
Figure 12. An illustration of the intrinsic and extrinsic toughening mechanisms in epoxy-carbon reinforced composites. Adapted with permission from Ref. Citation152. (a) SEM image of the fracture surface of unmodified epoxy polymer; photograph of the fracture surface of epoxy nanocomposites with (b1) 1.5 wt% aligned CNFs and (b2) 1.5 wt% aligned GNPs; SEM images of the fracture surface of epoxy nanocomposites containing (c) 1.5 wt% randomly-orientated GNPs; (d) 1.5 wt% aligned GNPs; (e) 1.5 wt% randomly-orientated CNFs; and (f) 1.5 wt% aligned CNFs.