Figures & data
Figure 1. Pathways through which ΔNp63α regulates keratinocyte senescence and ΔNp63α is regulated. (a) ΔNp63α regulates transcription of diverse genes involved in cell cycle. Consequently, keratinocyte senescence and its detrimental outcomes are prevented. (b) ΔNp63α transactivates multiple genes to activate Hh, Notch, and Wnt signaling pathways. In turn, stem cell self-renewal is maintained. (c) Various stresses can stimulate p38 MAPK or PI3K/Akt pathways, resulting in downregulation of ΔNp63α at the mRNA or protein level.
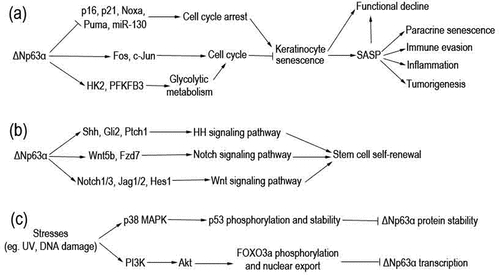
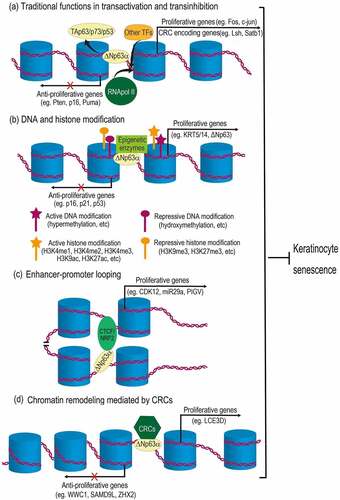
Figure 2. Mechanisms of ΔNp63α-mediated epigenetic regulation in preventing keratinocyte senescence. (a) As a traditional transcription factor, ΔNp63α binds to promoter regions, antagonizing its homologues (TAp63, p73 or p53), as well as recruiting other transcription factors (TFs) and RNA polymerase II (RNApol II), regulating genes related to cell cycle and chromatin remodeling complex (CRC). (b) ΔNp63α recruits diverse epigenetic enzymes (e.g., DNMT3A, KMT2D, HDAC1/2), leading to either active or repressive modification of DNA and histones. (c) ΔNp63α cooperates with CTCF or NRF2 to mediate enhancer-promoter looping. (d) ΔNp63α can also recruit CRCs (e.g., BAF, ACTL6A, SRCAP) to change chromatin configurations, which may either increase or decrease DNA accessibility for transcription machinery. Resultantly, proliferative genes are upregulated, while anti-proliferative genes are downregulated. Therefore, proliferation of keratinocytes is maintained and senescence is prevented.
Data availability statement
Not applicable.
