Figures & data
Figure 1. Lnc KCNQ1OT1 and MDR1 level were highly expressed in DOX-resistant BC cells, while miR-103a-3p level was lowly expressed. Note: a, IC50 values of BC cells and DOX-resistant BC cells were determined by MTT. b, cell proliferation was detected using clone formation. c, the apoptosis rate was measured with flow cytometry. d, lnc KCNQ1OT1, miR-103a-3p, and MDR1 expression was tested using qRT-PCR. e, western blot was performed to measure MDR1 expression.
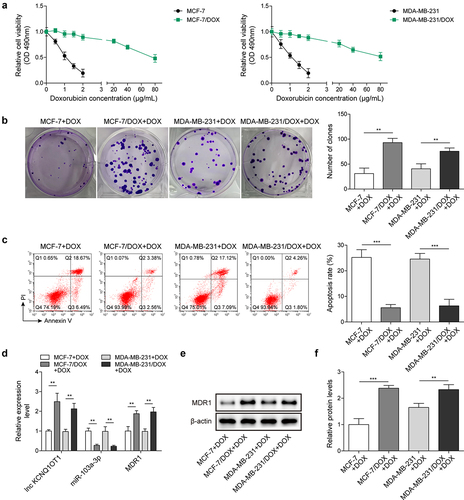
Table 1. IC50 values of different cell lines under DOX treatment.
Figure 2. Lnc KCNQ1OT1 depletion restrained DOX resistance in BC cells and DOX-resistant BC cells via elevating miR-103a-3p.Note: sh-lnc KCNQ1OT1 was transfected into DOX-resistant BC cells. a, qRT-PCR was employed to detect transfection efficiency and miR-103a-3p expression. b, cell viability was determined by MTT. c, the capability of cell proliferation was detected using clone formation. d, the apoptosis rate was measured with flow cytometry. e, western blot was performed to measure Bax, Bcl-2 expression. f, the mutual binding site between lnc KCNQ1OT1 and miR-103a-3p was predicted by starbase, and the interplay between lnc KCNQ1OT1 and miR-103a-3p was validated by dual-luciferase reporter gene assay and RIP assay.
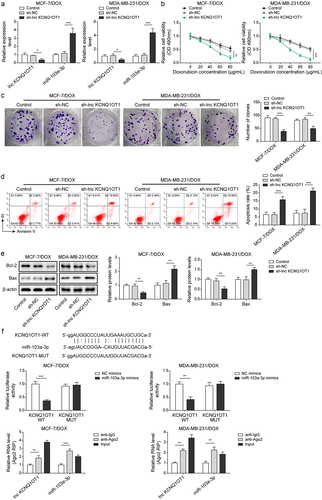
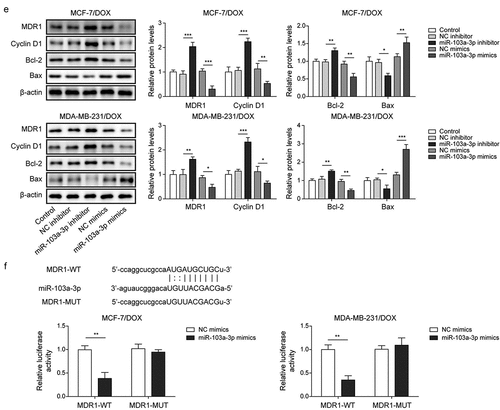
Figure 3. MiR-103a-3p overexpression/knockdown affected DOX resistance of BC cells and DOX-resistant BC cells through modulating MDR1.Note: DOX-resistant BC cells suffered miR-103a-3p mimics/inhibitor. a, qRT-PCR was employed to detect transfection efficiency and MDR1 expression. b, cell viability was determined by MTT. c, cell proliferation was detected using clone formation. d, the apoptosis rate and cell cycle were measured using flow cytometry. e, western blot was performed to measure MDR1, cyclin D1, Bax, and Bcl-2 expressions. f, the mutual binding site between miR-103a-3p and MDR1 was predicted by starbase, and the interplay between miR-103a-3p and MDR1 was consolidated by dual-luciferase reporter gene assay.
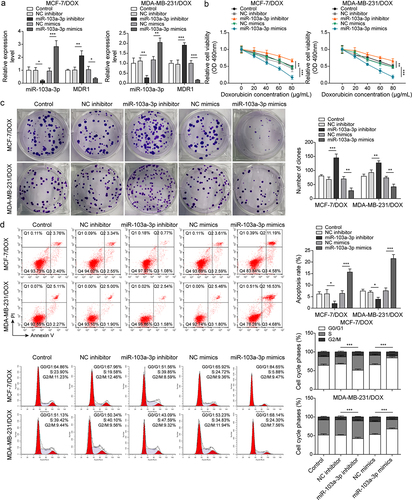
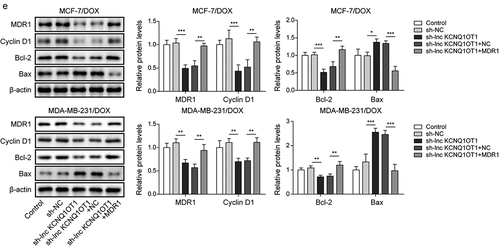
Figure 4. Lnc KCNQ1OT1 indirectly elevated MDR1 expression to promote DOX resistance of BC cells and DOX-resistant BC cells.Note: DOX-resistant BC cells were transfected with sh-lnc KCNQ1OT1 alone or along with oe-MDR1. a, qRT-PCR was employed to detect lnc KCNQ1OT1, miR-103a-3p, and MDR1 expressions. b, cell viability was determined by MTT. c, cell proliferation was detected using clone formation. d, the apoptosis rate and cell cycle were measured with flow cytometry. e, western blot was performed to measure MDR1, cyclin D1, Bax, and Bcl-2 expressions.
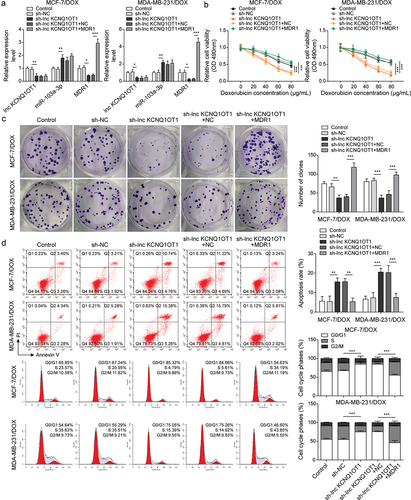
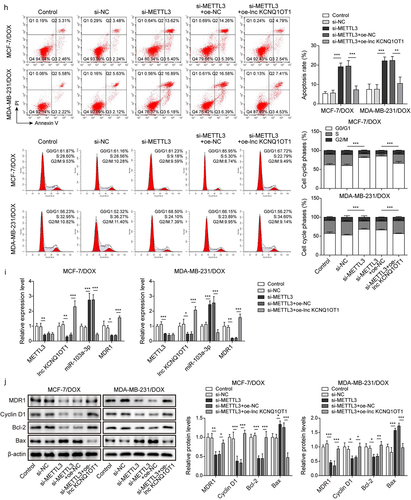
Figure 5. METTL3 modulated m6A modification of lnc KCNQ1OT1 to facilitate DOX resistance of DOX-resistant BC cells.Note: a, qRT-PCR was applied to examine METTL3 expression in MCF10A, MCF-7, MDA-MB-231, MCF‐7/DOX, and MDA-MB-231/DOX cells. si-METTL3 or oe-METTL3 was transfected into DOX-resistant BC cells. b, qRT-PCR was performed to measure METTL3 and lnc KCNQ1OT1 expression. c, qRT-PCR was applied to detect the stability of lnc KCNQ1OT1. d, MeRIP‐qPCR was applied to test lnc KCNQ1OT1 expression of m6A modification. e, RIP assay was adopted to evaluate the enrichment of lnc KCNQ1OT1 by anti-METTL3 in MCF‐7/DOX and MDA-MB-231/DOX cells. si-METTLE3 alone or along with oe-lnc KCNQ1OT1 were transfected into DOX-resistant BC cells. f, cell viability was determined by MTT. g, cell proliferation was detected using clone formation. h, the apoptosis rate and cell cycle were measured with flow cytometry. i, lnc KCNQ1OT1, miR-103a-3p, and MDR1 expressions were tested using qRT-PCR. j, western blot was performed to measure MDR1, cyclin D1, Bax, and Bcl-2 expressions.
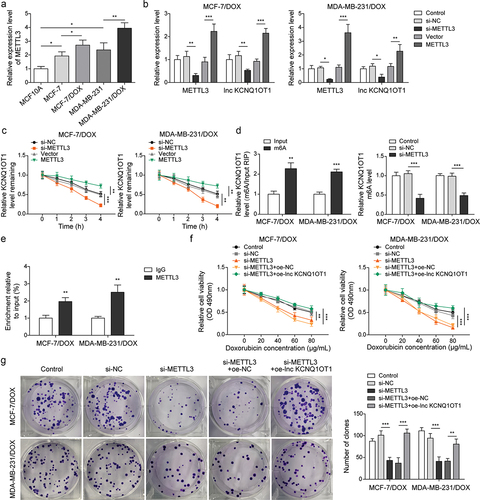
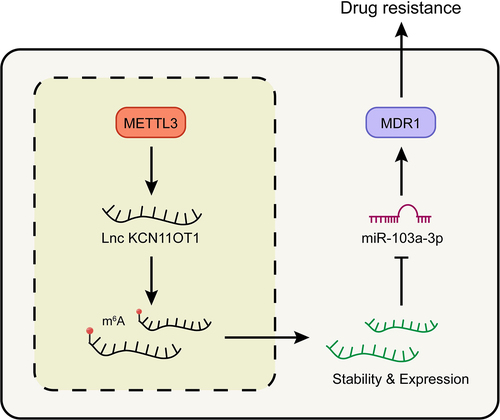
Figure 6. Schematic of molecular mechanism.Note: METTL3 promoted the expression of lncKCNQ1OT1 by regulating its m6A modification. While the up-regulated lncKCNQ1OT1 played the role of ceRNA in the targeted inhibition of miR-103a-3p, and MDR1, as the downstream molecule targeting miR-103a-3p, could enhance DOX resistance of breast cancer.