Figures & data
Figure 1. Serines S231 and S257 of AGL15 are phosphorylated in stage 15 floral receptacles. (A) AGL15 expressed as a hemagglutinin (HA) tag in stage 15 floral receptacles, driven by its native promoter, runs a multiple bands on 25 µM Phos-tag gels.Citation16 Mutating serines 231 and 257 to alanine reduces phosphorylated isoforms of AGL15. Exposures were chosen for each lane that scaled the 2 lanes to similar intensity for the lower, non-phosphorylated band. Expression was performed in the AGL15 null mutant, agl15-4.Citation18 (B) Quantified ratio of phosphorylated to non-phosphorylated AGL15. Three independent lines were assayed. *P < 0.05 (t-test). n = 3; SEM error bars shown. Floral receptacles (1 mm hand cut cross section of the base of the flower) were ground in liquid nitrogen and then crude protein extracts were prepared by mixing the ground tissue with 1x SDS sample buffer (1 receptacle per 12 ul SDS sample buffer). Phos-tag gel electrophoresis and Western blotting were performed as described.Citation16 Quantification of Western blots was performed with Image Lab software (Bio-Rad). Mutations to the previously described AGL15 native promoter construct were made via the QuikChange method (Agilent).Citation16
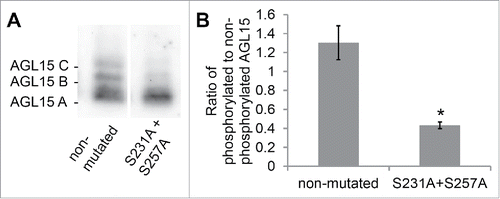
Figure 2. AGL15 phosphomimetics have similar mobility shifts as 35S-AGL15 from floral receptacles. AGL15 produced by the 35S promoter in floral receptacles runs as 2 bands on one day old 12% SDS-PAGE gels. The slower mobility band can be shifted to the faster mobility band by phosphatase treatment.Citation16 However, 35S-AGL15 expressed in mesophyll protoplasts runs as a single band. Mutating either S231 or S257 to aspartate reduces AGL15 mobility with the double substitution causing the biggest mobility shift similar to phosphorylated AGL15. The experiment was repeated twice with the same result. Protoplast preparation and transfection was performed as described.Citation19 Six hours after transfection, protoplasts were solubilized in SDS sample buffer. SDS-PAGE and Western blotting were performed as described.Citation16 35S-AGL15 floral stage 15 receptacle extract was prepare as described in the legend. cDNA from the previously described construct used to generate 35S-AGL15-double HA tagged plants was inserted into pRLT2 for protoplast studies and mutations were made via the QuikChange method (Agilent).Citation16
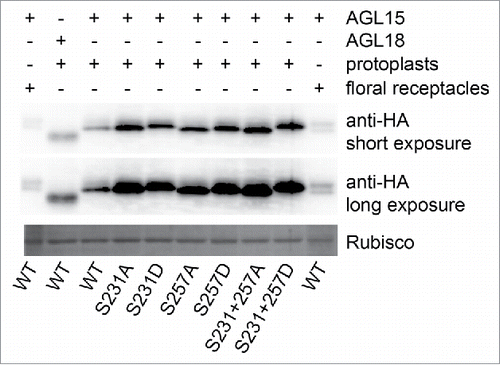
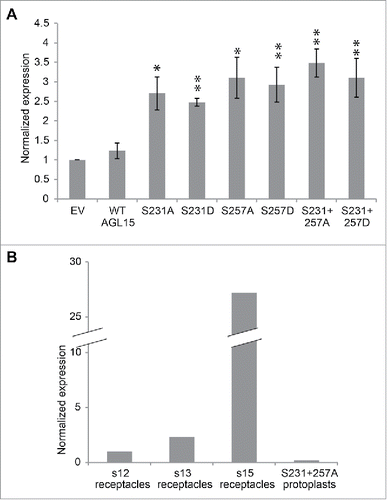
Figure 3. Mutating S231 and S257 of 35S-AGL15 alters HAESA expression in mesophyll protoplasts. (A) HAESA transcript accumulation in mesophyll protoplasts 6 hours after transfection with empty vector or 35S-AGL15 with indicated mutations. Protoplast were prepared as describes.Citation19 RNA was extracted from protoplasts with Trizol (Ambion). Quantitative-PCR was performed as described.Citation16 *P < 0.1, **P < 0.05 (t-test). n = 3; SEM error bars shown. (B) Relative expression of HAESA in floral receptacles and in transfected mesophyll protoplasts.