Figures & data
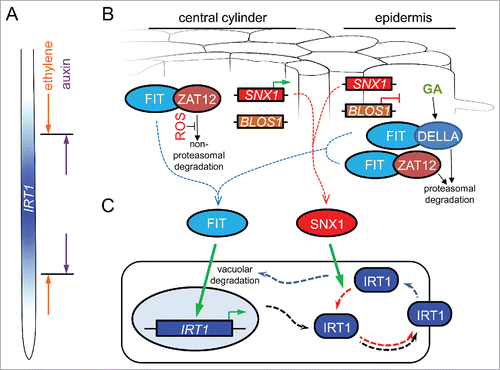
Figure 1. A hypothetical model for the cell-specific regulation of IRT1 expression and protein recycling under iron deficiency. (A) IRT1 is expressed in the early differentiation zone of the root. Its expression domain is defined by the opposing effects of the phytohormones ethylene and auxin. (B) In the central cylinder, FIT activity is inhibited through its interaction with ZAT12. In the absence of reactive oxygen species (ROS), ZAT12 is unstable and is degraded through a non-proteasomal pathway. This releases potentially active FIT. SNX1 expression is upregulated in this zone (green arrow), enhancing the cellular capacity to recycle SNX1 target proteins. In the epidermis, FIT can engage in protein complexes with ZAT12 but also with DELLA proteins, such as RGA. In these cells, ZAT12 undergoes proteasome-mediated degradation. Under iron deficiency, the phytohormone gibberellin (GA) promotes the proteasomal degradation of DELLA. Both events may be mediated through the COP9 signalosome (not depicted) and result in the release of potentially active FIT. The gene encoding the SNX1 interactor BLOS1, promoting the vacuolar degradation of membrane proteins, is downregulated in the epidermis (red blunt line), thereby increasing the SNX1 potential for protein recycling. (C) Under iron deficiency, active FIT in both central cylinder and the epidermis promotes the expression of IRT1. The IRT1 protein is targeted to the plasma membrane for iron uptake (black punctate arrows). Endocytosis (blue punctate arrows) may lead to IRT1 degradation in the vacuole. Alternatively, endosomal IRT1 may be recycled and sent back to the plasma membrane (red punctate arrows). Accumulation of active SNX1 promotes IRT1 stability and increased plasma membrane abundance.