Figures & data
Figure 1. Subcellular localization of MpRRB. (a) Thalli of the Mprrb knockout line and the same line carrying proMpRRB:MpRRB-Citrine. The genomic fragment containing the promoter and the coding regions of MpRRB was introduced into the binary vector pMpGWB307,Citation11 which was then transformed into the Mprrb knockout line having an F1 background generated by crossing Tak-1 and Tak-2. Thallus tips were cultured for 15 days. Arrowheads indicate gemma cups. Bars represent 1 cm. (b) Expression and protein localization of Citrine-fused MpRRB at the notch. Gemmae were cultured for five days, and harvested thalli were fixed and cleared. Images were obtained with the confocal microscope (FV1000, Olympus). The lower panels are enlarged images of the notch region. Fluorescence images of Citrine signals (left); brightfield images (middle); merged images (right). Arrowheads indicate apical notches. Bars represent 100 µm. (c) Protein localization of Citrine-fused MpRRB in Arabidopsis protoplasts. MpRRB-Citrine was expressed under the cauliflower mosaic virus 35S promoter in Arabidopsis protoplasts prepared from suspension cultured cells. Fluorescence image of Citrine signal (upper left); DAPI-stained nuclei (upper right); bright-field image (lower left); merged image (lower right). Bar represents 20 µm.
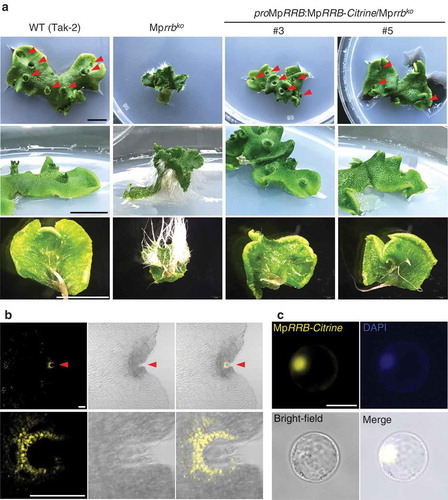
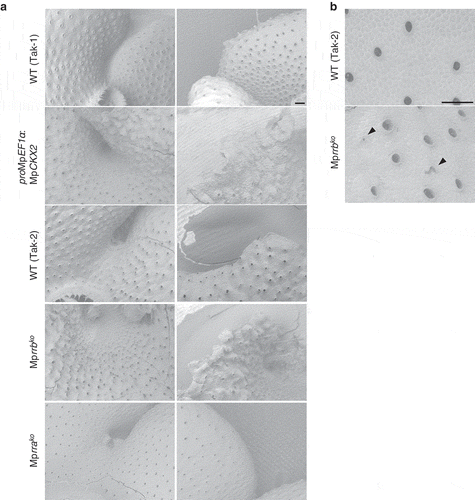
Figure 2. Scanning electron micrographs of the MpCKX2-overexpressing line and the knockout lines of MpRRA and MpRRB. (a) Apical notch regions (left) and thallus margins (right). The MpCKX2-overexpressing line and the knockout lines of MpRRA and MpRRB are male and female lines, which have Tak-1 and F1 background, respectively. (b) Magnified images of the dorsal side of thalli. Thallus tips were cultured for 14 days and observed with a scanning electron microscope TM3000 (hitachi high technologies). Arrowheads indicate air pores without a circular shape. Bars represent 200 µm.