Figures & data
Figure 1. Schematic representation of cold treatments and phenotype characterization of plants.
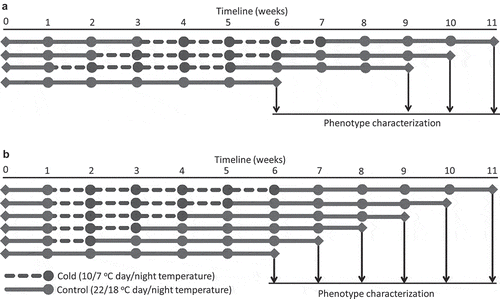
Figure 2. Prolonged cold exposure to juvenile seedlings enhances plant growth traits and shoot branching.
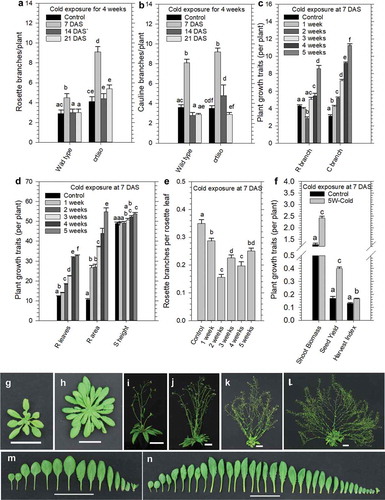
Figure 3. Prolonged cold exposure of juvenile plants enhanced growth and branching traits in mutants displaying a hyper-branched phenotype and stem cell maintenance of the shoot apical meristem. Juvenile wild type (WT) and mutants (carotenoid cleavage dioxygenase 7; ccd7, branched 1; brc1, set domain group 8; sdg8, and DNA methyl transferase 1; met1 were grown for 7 DAS under normal conditions and then transferred to the cold (10/7°C day/night cycle) for 5-weeks, after which they were returned to normal growth conditions until reproductive maturity. Growth traits measured include; a) number of rosette leaves, b) number of emerged rosette shoot buds (<5 mm), c) number of rosette branches (>5 mm), d) number of cauline branches, e) primary inflorescence stem height to the first silique, F) number of shoot buds per rosette leaf, g) number of branches per rosette leaf, H) number of cauline branches per cm stem height. Mean values display standard error of the means (n = 15). Denoted letters indicate statistical variation (p < .05) in growth attributes within treatment groups and across genotypes as determined by a Two-Way ANOVA using a Holm-Sidak post hoc multiple comparison.
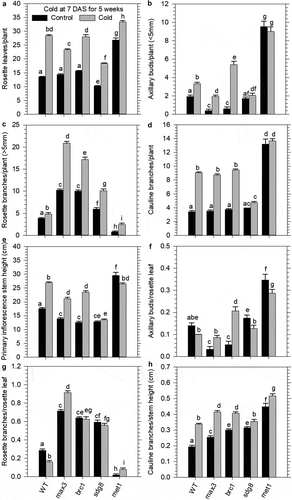
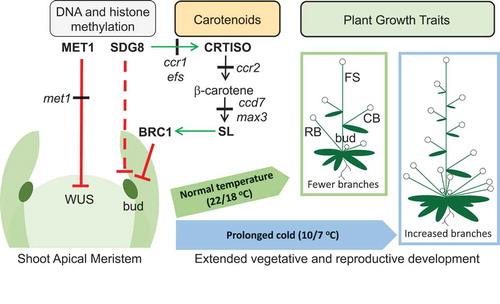
Figure 4. Model showing how exposure of juvenile seedlings to prolonged cold enhances shoot branching in Arabidopsis. DNA METHYLTRANSFERASE 1 (MET1) negatively regulates WUSCHEL (WUS), which maintains the stem cell niche of the shoot apical meristem (SAM) in an undifferentiated state. The met1 mutant impairs CG context-dependent DNA methylation, enhancing WUS expression in the SAM that enhances the formation of axillary buds.Citation40,Citation49 Leaf primordia and axillary bud differentiation in the axillary meristem (AM) is programmed during juvenile seedling development (<12 days after stratification; DAS), after which the SAM transitions into a inflorescence meristem (IM; >14 DAS).Citation4 Upon flowering the primary inflorescence stem (FS; floral bolt) emerges from the IM. A cauline branch (CB) and leaf emerges at nodes along the primary inflorescence stem. SET DOMAIN GROUP 8 (SDG8) maintains CAROTENOID ISOMERASE (CRTISO) gene expression and accumulation of β-carotene, a substrate required for biosynthesis of strigolactone (SL).Citation23 SL regulates BRANCHED 1 (BRC1), which represses axillary bud outgrowth, that would otherwise emerge into a rosette branch (RB).Citation24,Citation50 sdg8 (ccr1) and crtiso (ccr2) mutants may impair SL biosynthesis,Citation51 while the loss-of-function in CAROTENOID CLEAVAGE DIOXYGENASE 7 mutant (ccd7) prevents SL biosynthesis.Citation21 Negative and positive regulation are indicated by solid red and green arrowed lines, respectively. Black arrowed lines indicate carotenoid biosynthesis steps in pathway. The red dashed lines indicate a presumed function of SDG8 in controlling histone methylation in the SAM.