Figures & data
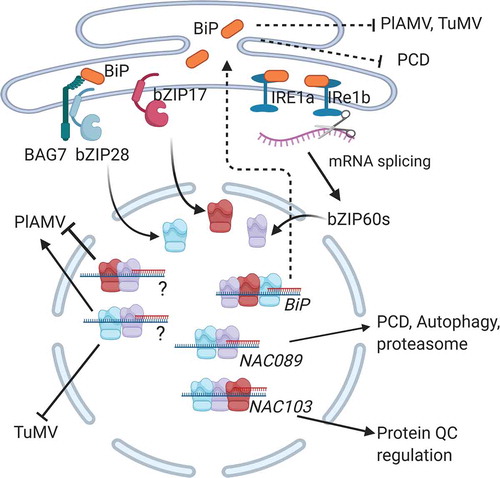
Figure 1. UPR mediated suppression of plant virus infection. The IRE1a/IRE1b-dependent, bZIP28/bZIP17 dependent pathways are routes leading to transcriptional activation of genes that regulate pro-survival and pro-death events. BiP is an ER resident molecular chaperone that is a master regulator of these ER stress transducers to block their activation. BiP also functions in the ER lumen to facilitate protein folding in an ATPase dependent manner. The bZIP60, bZIP28, and bZIP17 bind to the BiP promoter and increasing its expression to enhance protein folding in the ER lumen and to serve as part of a negative feedback loop to block further activation of these stress transducers in the ER. IRE1a/IRE1b endonuclease activity splice the bZIP60 mRNA to produce a transcription factor that is mobilized to the nucleus. NAC089 and NAC103 are activated to regulate programmed cell death and enhance protein quality control. bZIP60 and bZIP17 form complexes that activate unknown genes that limit PlAMV infection. The bZIP60 and bZIP28 activate unknown genes that support PlAMV infection but limit TuMV infection. Overexpression of BiP or enhancing protein folding capacity through TUDCA limits virus infection and suppressed PCD