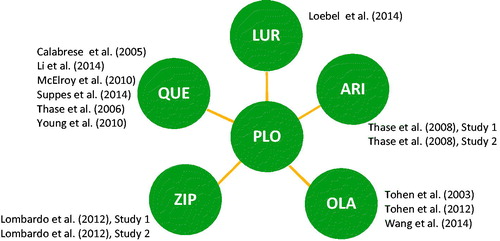
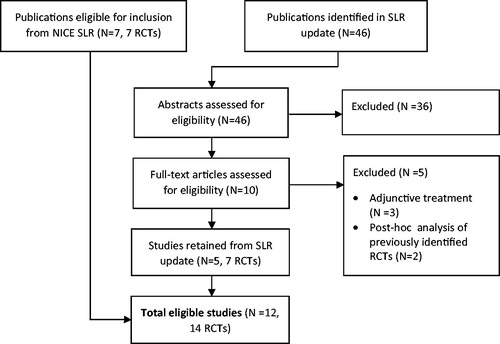
Figures & data
Table 1. Eligibility criteria for assessment of study inclusion.
Table 2. Study design and patient baseline characteristics.
Table 3. Forest plots for base-case analysis of efficacy outcomes.
Table 4. Forest plots for base-case analysis of tolerability outcomes.
Appendix Table 1. Base-case results for the efficacy outcomes of change from baseline in MADRS score, CGI-BP-S score, response and remission.
Appendix Table 2. Base-case results for the tolerability outcomes of weight change, somnolence, extrapyramidal symptoms and overall discontinuation.
Appendix Table 3. Sensitivity analysis results for the efficacy outcomes of change from baseline in MADRS score, CGI-BP-S score, response and remission.
Appendix Table 4. Sensitivity analysis results for the tolerability outcomes of weight change from baseline, somnolence, extrapyramidal symptoms and overall discontinuation.
Appendix Table 5. Sensitivity analysis results for the efficacy and safety outcomes (excluding Wang et al. (Citation2014) from the base-case analysis).
Appendix Table 7. Sensitivity analysis results for the tolerability outcomes (excluding Wang et al. (Citation2014) from the base-case analysis).