Figures & data
Figure 1. Schematic representation of GABAA receptors (GABAARs) as heteropentameric complexes, made up of a combination of 19 subunits, delimiting a chloride-permeable channel when activated by at least two molecules of GABA. Extracellular sites for benzodiazepines and etifoxine, two anxiolytics, are indicated on the top view representation of the receptor channel, composed in the vast majority by αβγ subunits.
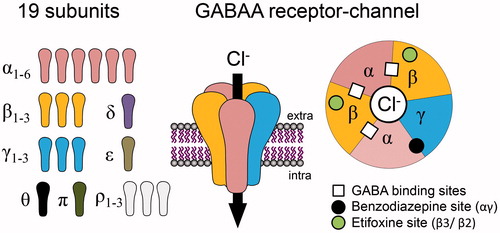
Table 1. List of some ligands exerting a positive modulation on GABAAR function. Binding sites, if identified, are indicated in the table with their specific properties. In the case of steroids, access to the binding site is presumed to be intracellular or via membrane lateral mobility. All other compounds bind to extracellular binding sites.
Figure 2. Simplified view of neurosteroidogenesis focussing on the synthesis of 3α-reduced neurosteroid anxiolytics targeting GABAARs. This production is fully ensured by nerve cells if cholesterol is used as a precursor or can be made possible by using circulating sex and stress steroids, if they reach nerve cells. Note that 3α-reduced neurosteroids are likely to reach their GABAAR binding sites by lateral membrane diffusion. Cholesterol transport by translocator protein (TSPO) has been shown to be stimulated by several compounds including etifoxine, which also exerts an allosteric modulation of GABAARs. DHDOC: dehydro-deoxycorticosterone; DHP: dihydroprogesterone; DHT: dihydrotestosterone; DOC: deoxycorticosterone; THDOC: tetrahydrodeoxycorticosterone; p450scc, cytochrome p450 enzyme side chain cleavage; 5αR, 5α-reductase; 3α-HSOR, 3α-hydroxysteroid dehydrogenase.
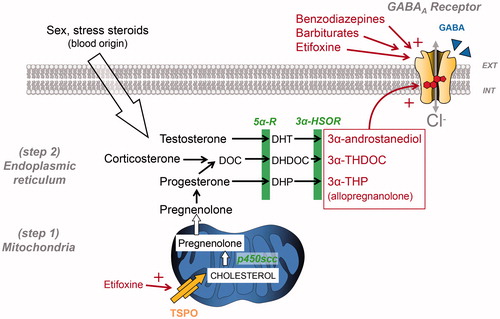
Table 2. Summary of the effects of benzodiazepines, either clinically relevant or adverse side effects, and the presumed α subunit composition of GABAARs (Adapted from (Tan et al. Citation2011)). Note that α4 or α6 subunit-containing GABAARs containing are not shown since they are benzodiazepine insensitive.
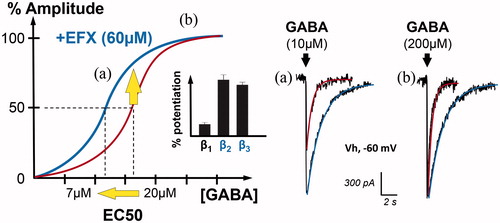
Figure 3. Positive allosteric modulation of GABAAR currents by the anxiolytic etifoxine (EFX). Graph on the left illustrates the GABAAR current amplitude (in %) when spinal neurons are submitted to increasing concentrations of GABA (red). In the presence of etifoxine (EFX, 60 µM), the dose–response curve is shifted to the left, indicating an increase in the apparent affinity for GABA. Indeed, the concentration of GABA inducing GABAAR-mediated current of half the maximal amplitude (EC50) is reduced from 20 to 7 µM. Bar chart in inset indicates that this modulation is more selective for GABAAR containing β2 and β3 subunits (adapted from (Hamon et al. Citation2003)). Another representation of this effect is shown on the right with representative traces. Etifoxine increased the amplitude and duration of GABAAR-mediated currents evoked by non-saturating concentration of GABA (a, 10 µM). In the case of saturating concentration (b, 200 µM), EFX only increased the duration of the current, as predicted by the dose–response curve.