Figures & data
Table 1. Experimental conditions for suspension copolymerization of GDGDA with GDMA.
Table 2. The physical properties of ingredients used in the suspension polymerization system.
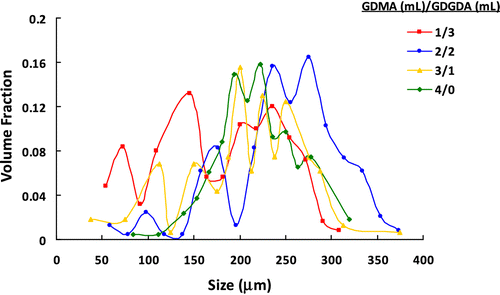
Table 3. The effect of GDMA/GDGDA volume ratio on the microbead yield, average size, and size distribution of poly(GDMA-co-GDGDA) beads.
Figure 2 Optical micrographs of poly(GDMA-co-GDGDA) beads synthesized with different GDMA/GDGDA ratios. GDMA/GDGDA ratios (mL/mL) are: (A) 1/3, (B) 2/2, (C) 3/1 and (D) 4/0. Original magnification: 125×.
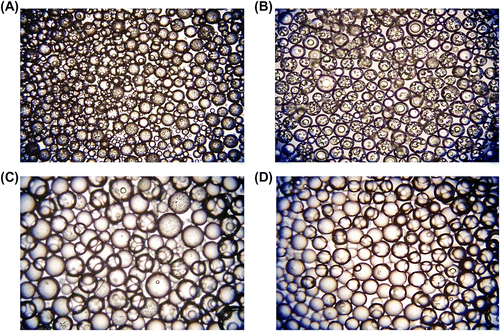
Figure 3 ESRs of poly(GDMA-GDGDA) gel beads synthesized with different GDMA/GDGDA volume ratios at different pHs.
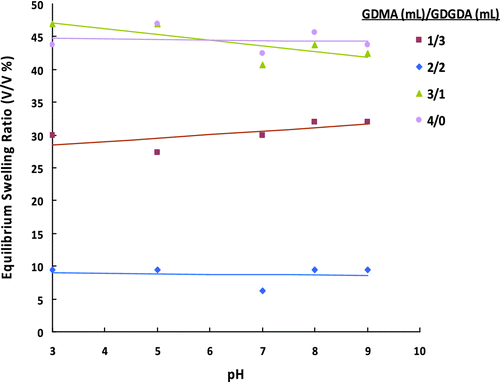
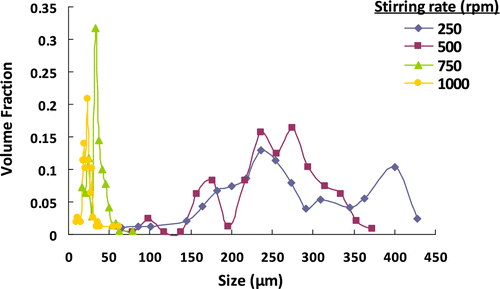
Table 4. The effect of stirring rate on the microbead yield, average size, and size distribution of poly(GDMA-co-GDGDA) beads.
Figure 5 Optical micrographs of poly(GDMA-co-GDGDA) beads with different stirring rates. Stirring rates: (A) 250, (B) 500, (C) 750, and (D) 1000 rpm, respectively. Original magnification: 125×.
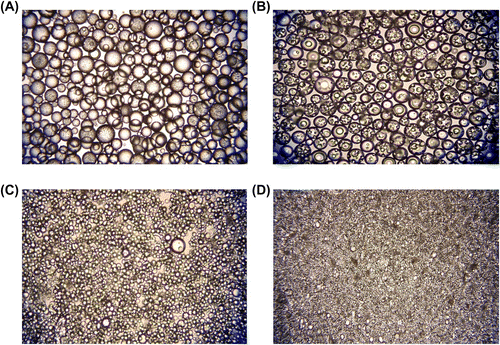
Figure 6 ESRs of poly(GDMA-co-GDGDA) gel beads synthesized with different stirring rates at different pH values.
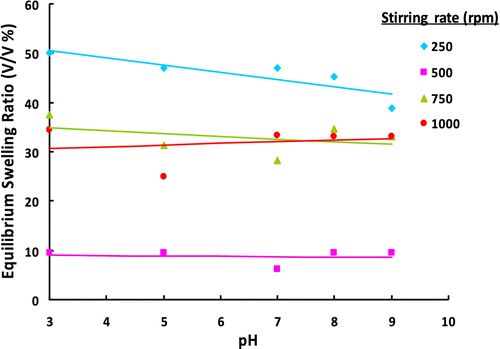
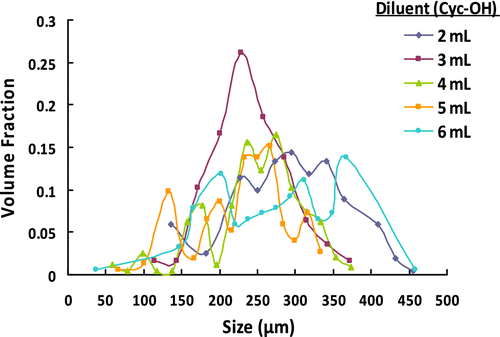
Table 5. The effect of amount of diluent on the microbead yield, average size, and size distribution of poly(GDMA-co-GDGDA) beads.
Figure 8 Optical micrographs of poly(GDMA-co-GDGDA) beads synthesized with different amounts of diluent. Amounts of diluent: (A) 2, (B) 3, (C) 4, and (D) 5 mL respectively. Original Magnification 125×.
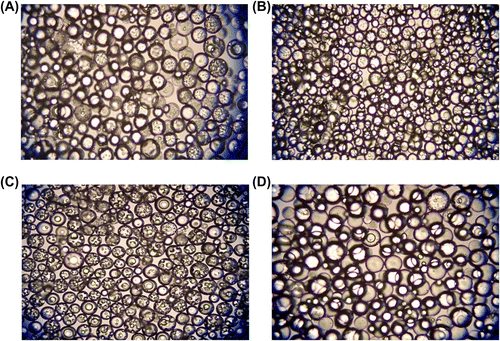
Figure 9 ESRs of poly(GDMA-co-GDGDA) gel beads synthesized with different amounts of diluent at different pH values.
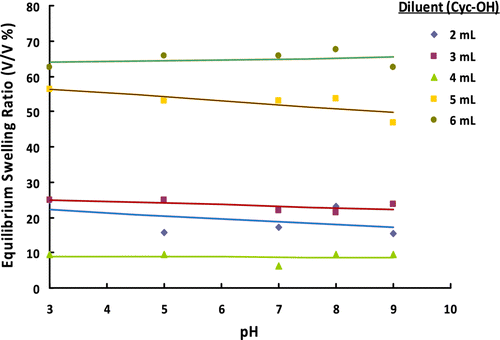
Table 6. The effect of functional monomer type on the microbead yield, average size, and size distribution of terpolymer beads including GDMA, GDGDA, and functional monomer.
Figure 10 The effect of functional monomer type on the size distribution of terpolymer microbeads including GDMA, GDGDA, and functional monomer.
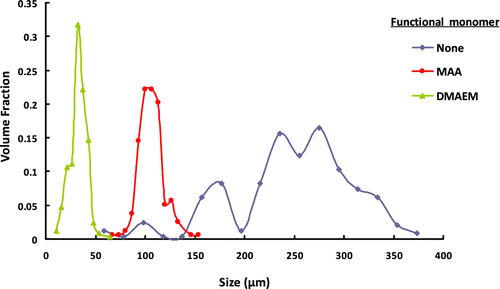
Figure 11 Optical micrographs of terpolymer beads synthesized with different functional monomers. Functional monomers: (A) None, (B) MAA, and (C) DMAEM. Original Magnification 125× for (A) and (B) and 500× for (C).
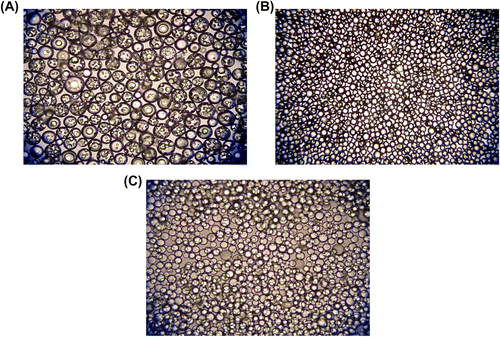
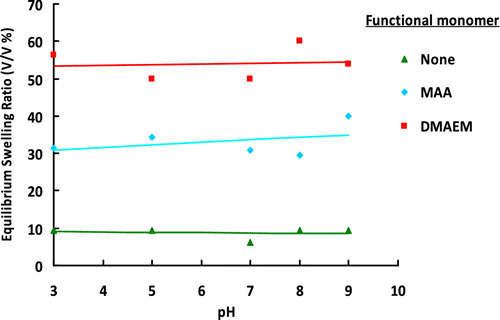
Figure 12 ESRs of terpolymer gel beads synthesized with different functional monomers at different pH values.