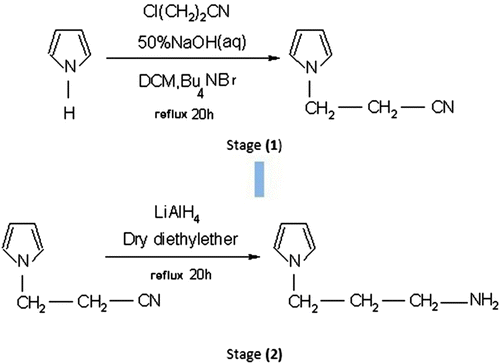
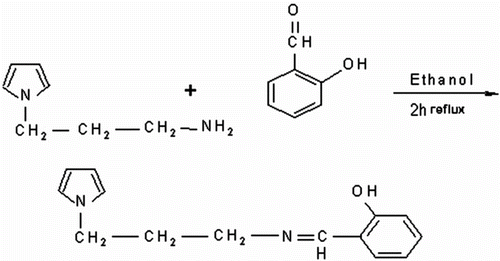
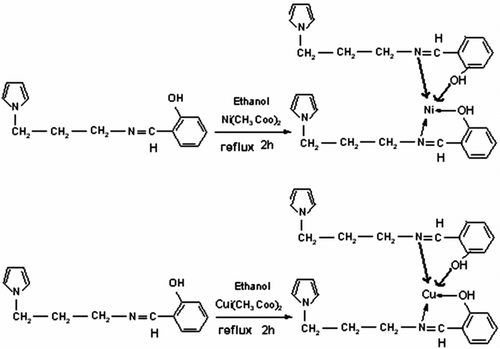
Figures & data
Figure 1 FT-IR spectra of (a) 1-cyanoethylpyrrole, (b) N-3-aminopropylpyrrole, (c) N3APS, (d) complex of N3APS with Ni(II), and (e) complex of N3APS with Cu(II).
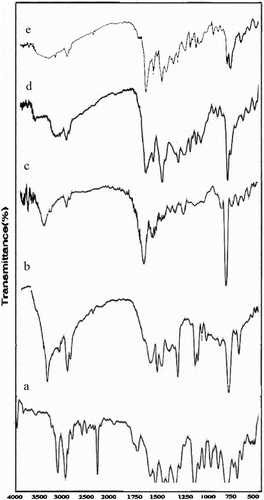
Figure 2 FT-IR spectra of (a) polypyrrole, (b) PN3APS, (c) copolymer N3APS/Py (70/30), (d) copolymer of N3APS/Py (60/40), and (e) copolymer of N3APS/Py (50/50).
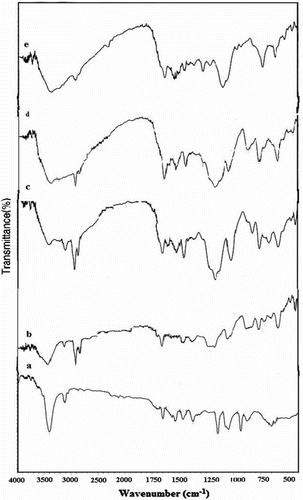
Table 1. Metal absorption ability of PN3APS.
Figure 6 SEM of (a) PN3APS synthesized by interfacial polymerization, (b) copolymer of Py/ N3APS (30/70) synthesized by interfacial polymerization, (c) PN3APS synthesized by ultrasonic method, and (d) copolymer of Py/ N3APS (30/70) synthesized by ultrasonic method.
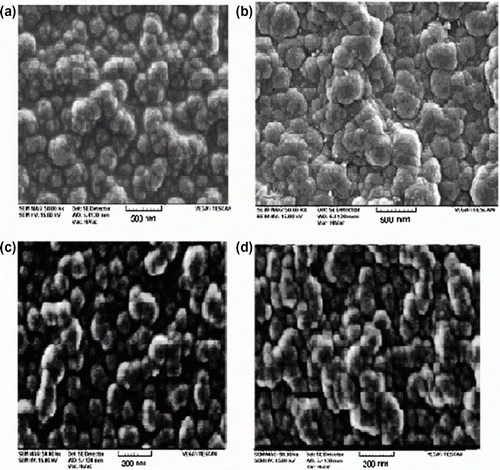
Table 2. Electrical conductivity (S cm−1) of PN3APS and its copolymers with various molar ratios of Py.
Table 3. Solubility of PN3APS and its copolymers with various molar ratios of Py:Schiff base.
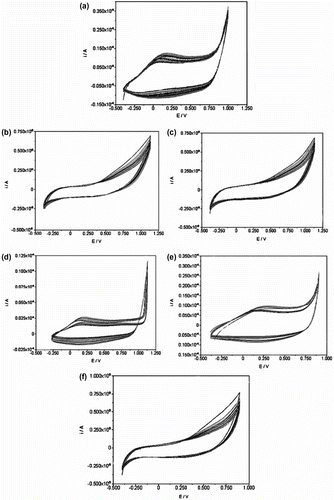
Figure 7 Cyclic voltamograms of (a) polypyrrole, (b) copolymer of Py/N-3-aminopropylpyrrole-samlicylaldehyde (10/90), (c) copolymer of Py/N-3-aminopropylpyrrole-salicylaldehyde (25/75), (d) copolymer of Py/N-3-aminopropylpyrrole-salicylaldehyde (50/50), (e) copolymer of Py/N-3-aminopropylpyrrole-salicylaldehyde (75/25), and (f) Poly(N-3-aminopropyl Py-salicylaldehyde).