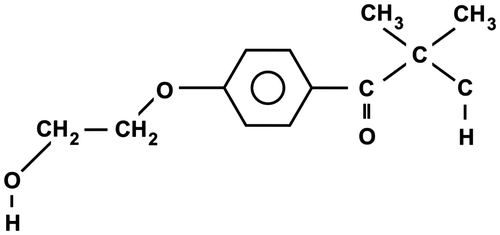
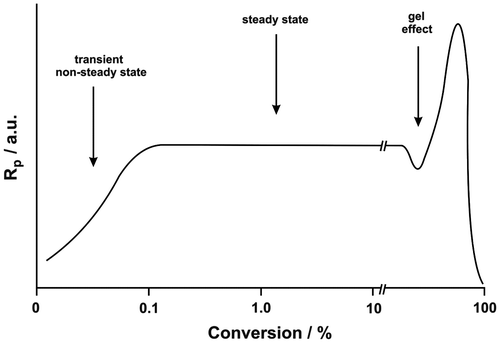
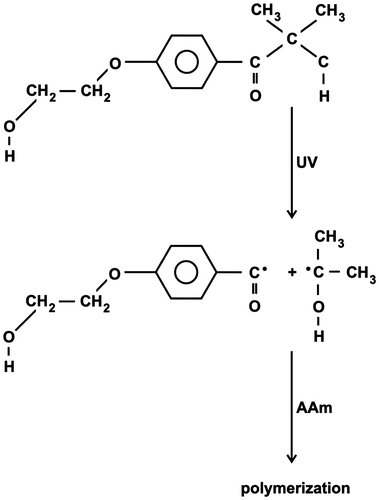
Figures & data
Figure 6. Variation of the rate of photoinduced solution polymerization of AAm with conversion and bubbling time. Recipe: 1.5 g AAm, 27 g H2O, 0.0515 g DAR, 1) bubbling time = 0 min, 2) bubbling time = 3 min.
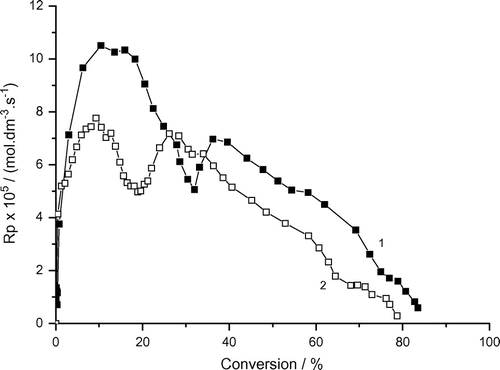
Figure 5. Variation of the rate of photoinduced micellar polymerization of AAm with conversion and bubbling time. Recipe: 1.5 g AAm, 27 g H2O, 0.36 g Tw60, 0.1030 g DAR, 1) under air, 2) under argon.
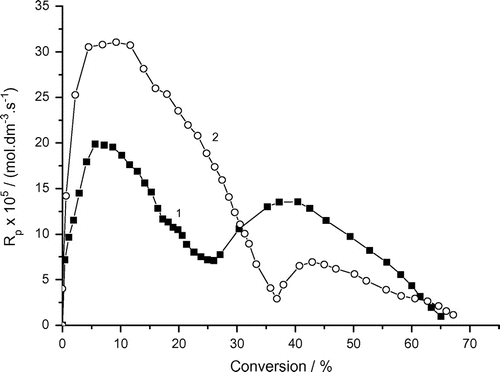
Figure 4. Variation of monomer conversion of photoinduced solution polymerization of AAm with reaction time and bubbling time. Recipe: 1.5 g AAm, 27 g H2O, 0.0515 g DAR, 1) bubbling time = 0 min, 2) bubbling time = 3 min.
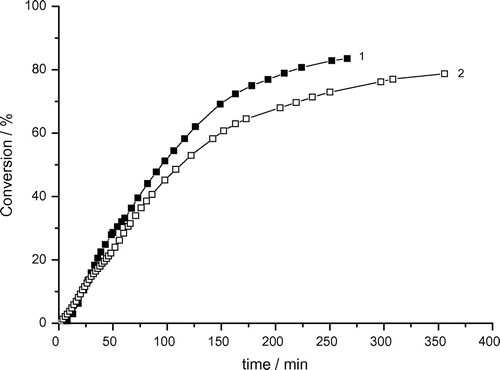
Figure 3. Variation of monomer conversion of photoinduced micellar polymerization of AAm with reaction time and bubbling time. Recipe: 1.5 g AAm, 27 g H2O, 0.36 g Tw60, 0.1030 g DAR, 1) bubbling time = 0 min, 2) bubbling time = 3 min.
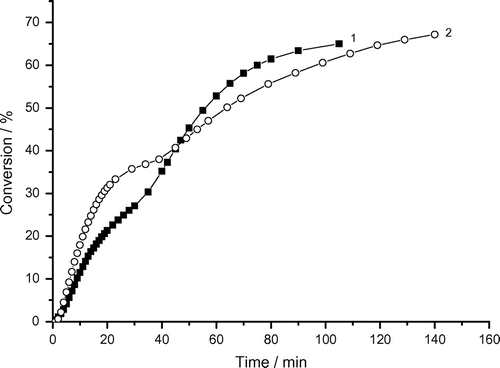
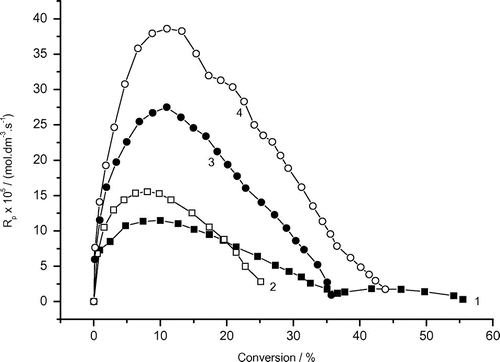
Figure 8. Variation of monomer conversion of photoinduced polymerization of AAm with reaction time and AAm concentrations. Recipe: 27 g H2O, 0.504 g Tw85, 0.103 g DAR 1) 0.5 g AAm, 2) 1.0 g AAm , 3) 1.5 g AAm, 4) 2.0 g AAm.
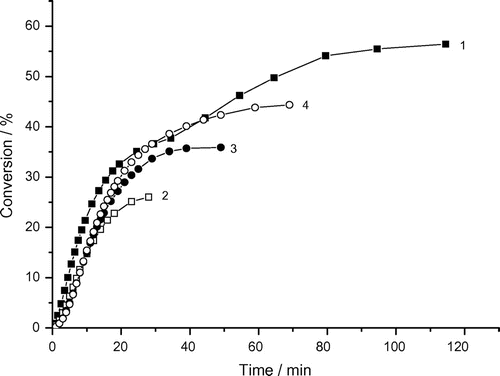
Figure 7. Variation of monomer conversion of photoinduced polymerization of AAm with reaction time and DAR concentrations. Recipe: 1.5 g AAm, 27 g H2O, 1) 0.01285 g DAR, 2) 0.02575 g DAR, 3) 0.0515 g DAR, 4) 0.1030 g DAR, 5) 0.2060 g DAR (under argon).
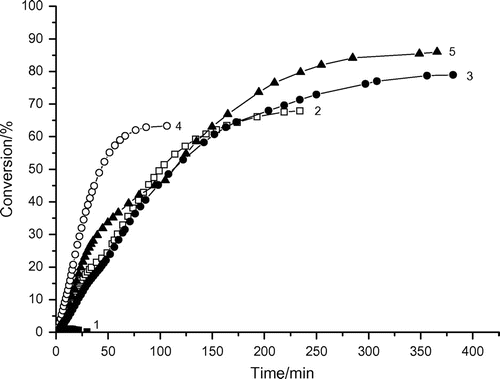
Table 1. Variation of kinetic parameters with the DAR concentration in the photoinitiated micellar polymerization of AAm.
Table 2. Variation of kinetic parameters with the DAR concentration in the photoinitiated micellar polymerization of AAm (SDS runs) with AAm concentration.