Figures & data
Figure 13. Optical absorption and photoluminescence spectra of pristine and annealed thin films at 50 and 70 °C of the synthesized polymers (A) P1 and (B) P4.
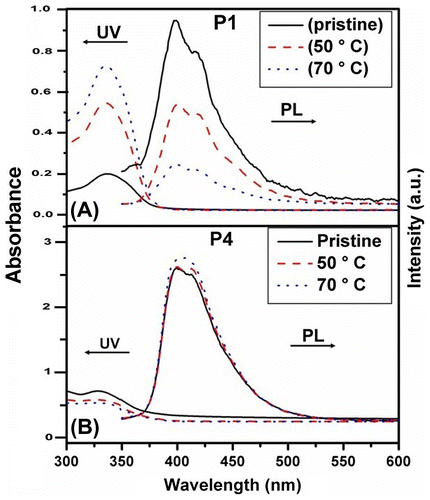
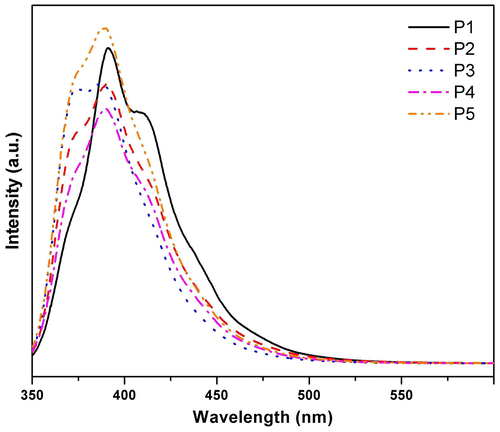
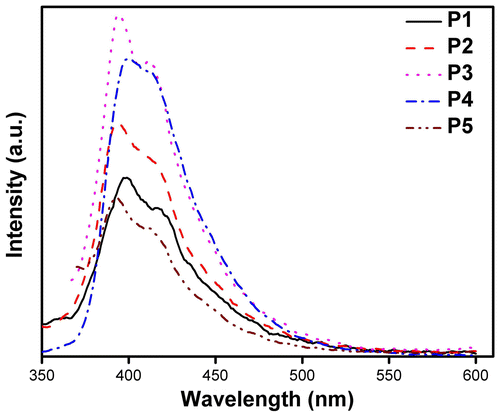
Figure 14. Photoluminescence spectra of pristine and annealed thin films of the synthesized polymers P1–P5.
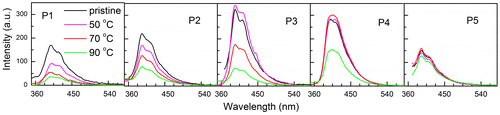
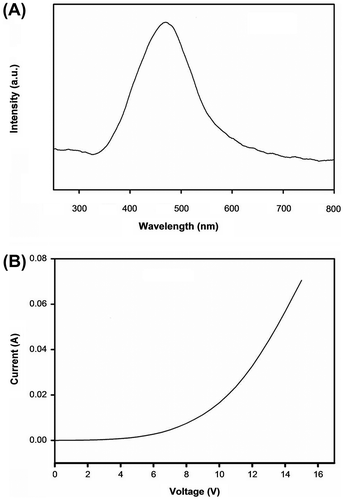
Figure 15. (A) Electroluminescence spectra of P5 using a single-layer cell with PEDOT:PSS-coated ITO anode and aluminum cathode and (B) the I–V characteristics of the PLED device.