Figures & data
Table 1. Overview of the synthesized monofunctional compounds 3a1–4 and 3b1–4 and their dimerization behavior.
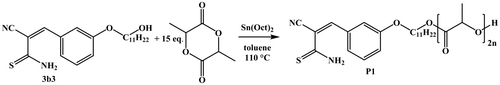
Figure 2. 1H NMR spectra of fraction 1, fraction 2, and fraction 2 after two weeks in solution of 3b1 in (CD3)2CO.
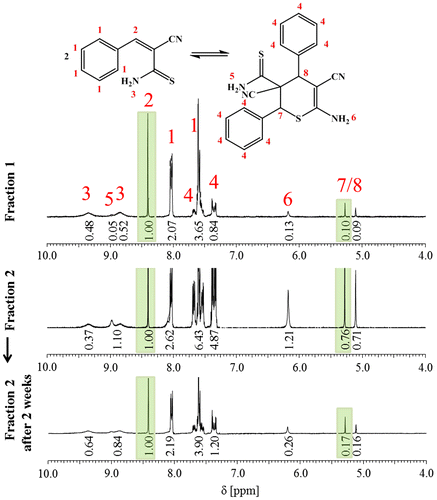
Figure 3. ESI-TOF mass spectrum of 3b4 with peak assignment and exemplary overlays of the measured and calculated isotopic patterns (monomeric and dimeric species).
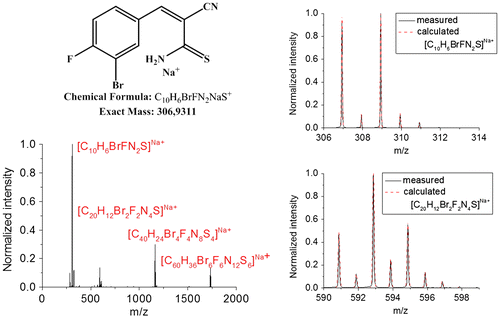
Figure 4. Equilibration study of 3b4. Data for the rHDA reaction obtained from (CD3)2CO solutions at 25 °C, data for the HDA reaction at 25 °C obtained after annealing at 70 °C in DMSO-d6 (left) and rHDA reaction at 50 °C in (CD3)2CO (right).
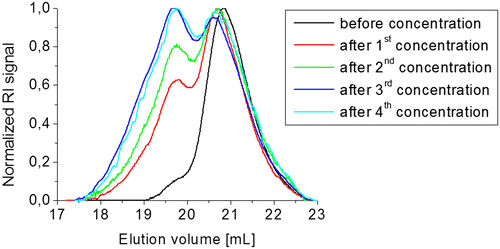
Figure 6. Study of the equilibrium of compound 3b5 at different concentrations in CH2Cl2, (a) directly after dissolving, (b) after 1 d in solution, (c) after 2 d in solution and (d) after 4 d in solution.
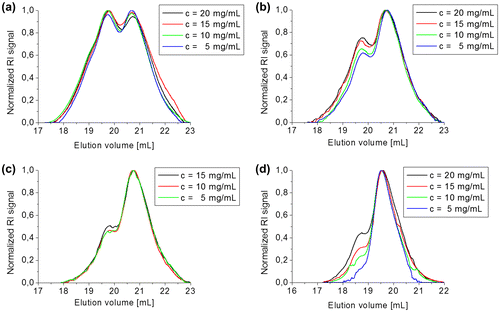
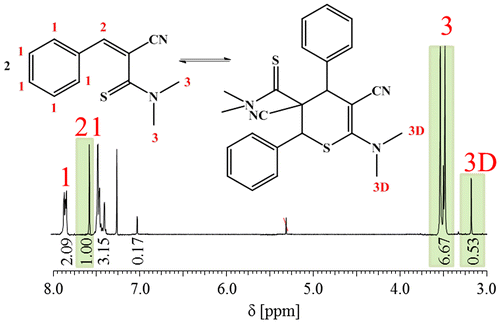
Figure 7. 1H NMR spectrum of 3b5 in CDCl3. For clarity, only the protons and the corresponding peaks that were used for the calculation of the average DP are labelled.
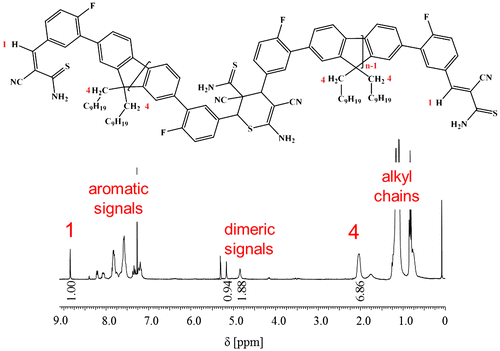
Figure 9. 3D contour plot from the SEC measurement of 3b5 with DAD. The corresponding RID signal is plotted on the right-hand side for comparison.
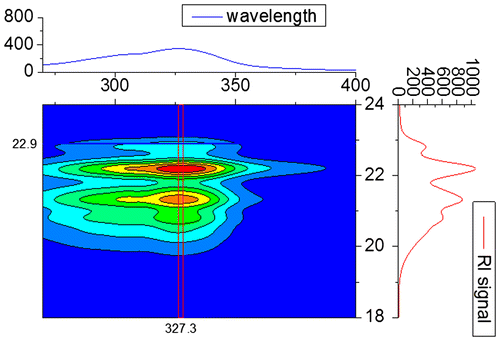
Figure 10. Characterization of P1 via mass spectrometry. Full MALDI-TOF mass spectrum (left) and overlay of calculated and measured isotopic patterns from ESI-TOF MS analysis (right).
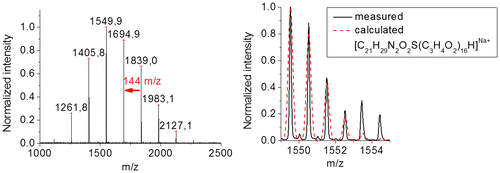
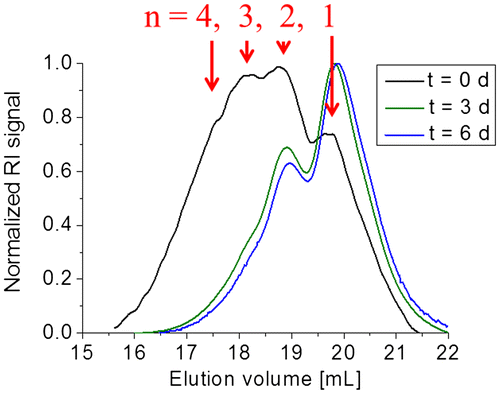
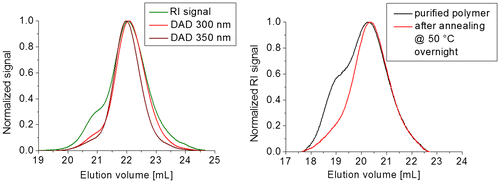
Figure 11. SEC measurement of P1 with RID and DAD (at 300 and 350 nm, the complete contour plot including other is depicted in the supporting information) (left) and SEC traces of P1 before and after annealing at 50 °C (right).
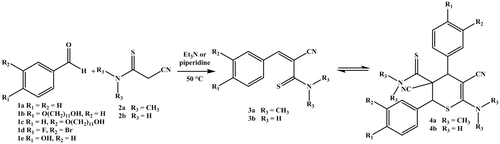
Scheme 1. Schematic representation of the synthesis of the 3-aryl-2-cyanothioacryl-N,N-dimethylamides (3a) and 3-aryl-2-cyanothioacrylamides (3b) as well as the dimerization via HDA reaction.
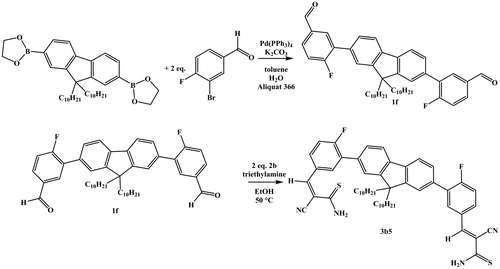
Scheme 2. Schematic representation of the synthesis of compound 1f via suzuki cross-coupling and subsequent Knoevenagel reaction to yield 3b5.