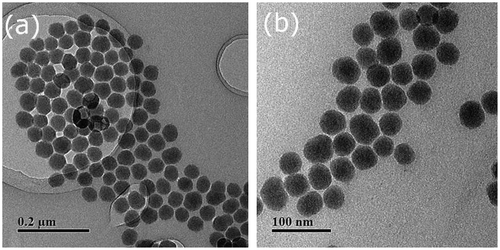
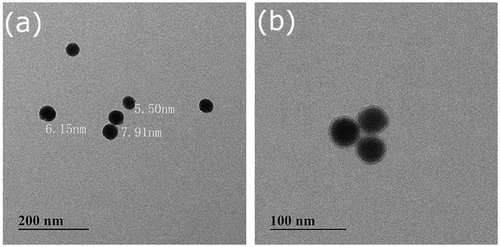
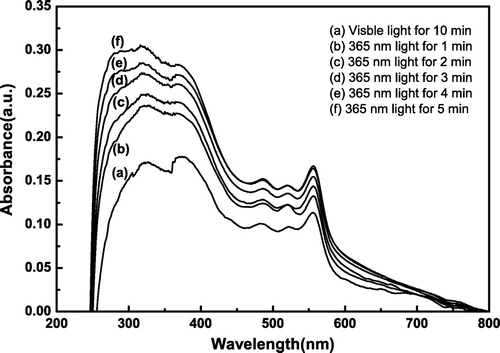
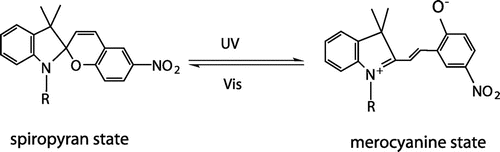
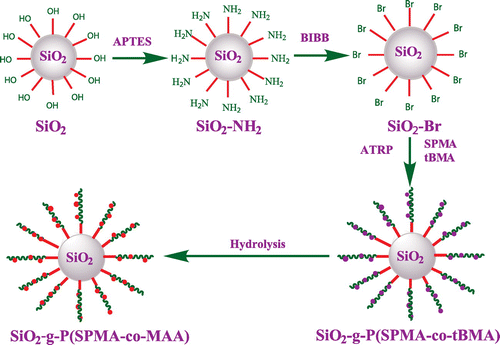
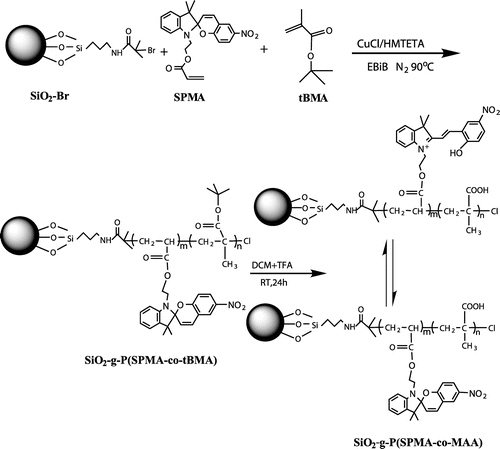
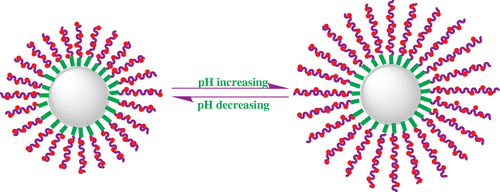
Figures & data
Figure 3. EDS spectrograms of (a) bare silica nanoparticles and (b) hybrid silica nanoparticles coated with P(SPMA-co-MAA).
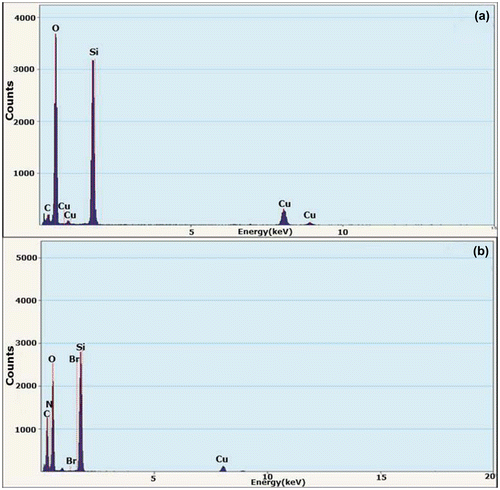
Figure 4. FT-IR spectra of (a) bare silica nanoparticles and (b) hybrid silica nanoparticles coated with P(SPMA-co-MAA).
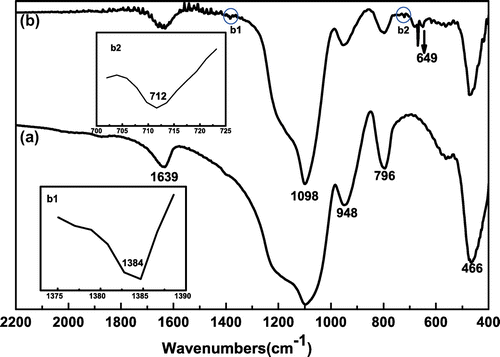
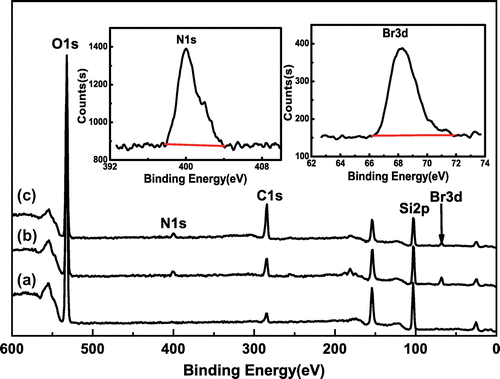
Table 1. Surface elements composition of SiO2, SiO2–Br, and SiO2-P(SPMA-co-MAA).
Figure 6. High-resolution XPS spectra and curve fitting of O1s of (a) SiO2 and (b) SiO2-P(SPMA-co-MAA).
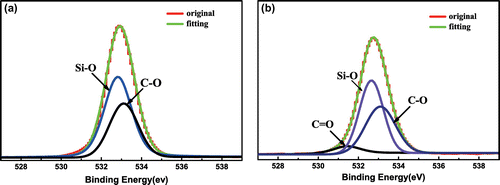
Table 2. O1s high-resolution XPS analysis of SiO2 and SiO2-P(SPMA-co-MAA).
Figure 7. The thermogravimetric analysis of (a) SiO2, (b) SiO2–NH2, (c) SiO2–Br, and (d) SiO2-P(SPMA-co-MAA).
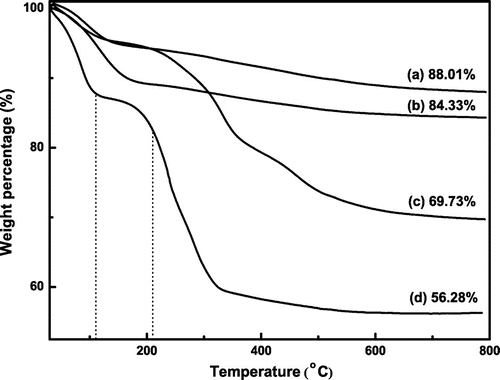
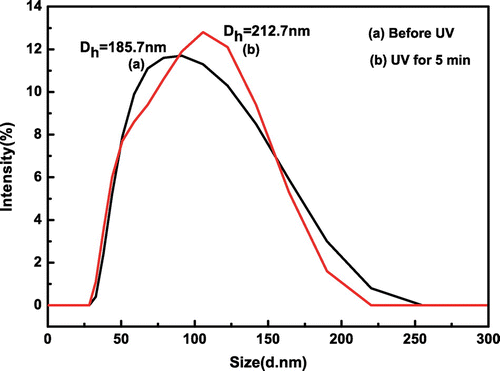
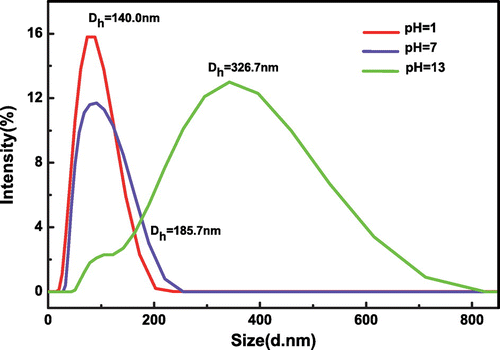
Figure 9. The hydrodynamic diameters distributions of SiO2-g-P(SPMA-co-MAA) under different light conditions.