Figures & data
Table 1. Composition, abbreviation, and Ti content of the prepared catalysts.
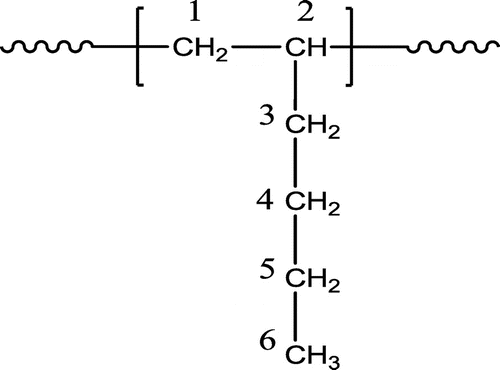
Figure 3. Expanded side chain methylene (C3) resonance patterns of PHs synthesized using Cat B, Cat C, and Cat D.
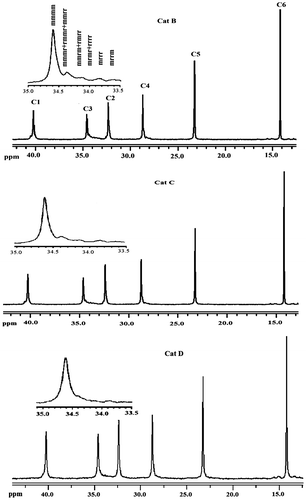
Figure 4. Expanded side chain methylene (C3) resonance patterns of poly(1-hexene) polymerized using Cat D in 25 °C.
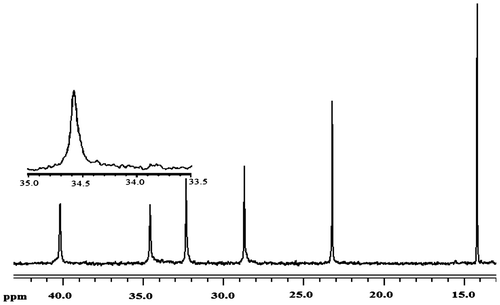
Figure 6. DSC curves of PHs obtained from (a) Cat C, Cat D, and Cat E in Tg region and (b) Cat C in Tm region.
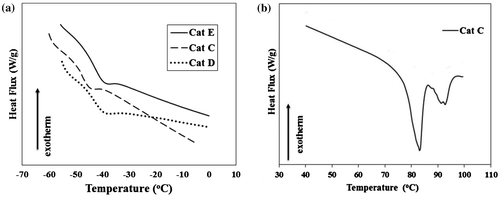
Figure 9. Employed models for the simulation of propene insertion into Ti-iBu active center. (a) MgCl-UD as undoped catalyst, (b) MgCl-D1 and (c) MgCl-D2 as doped catalyst models.
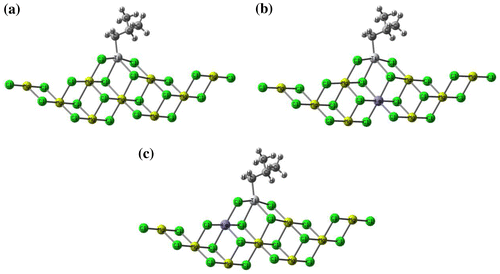
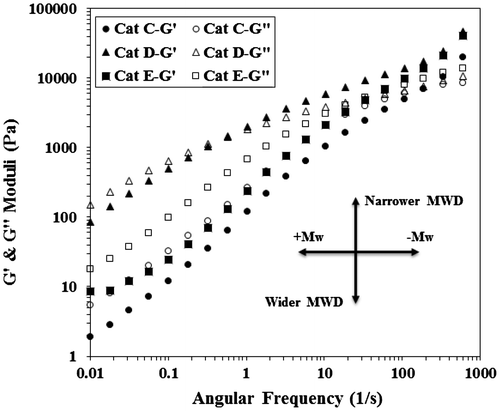
Figure 11. Storage and loss modulus versus frequency for poly(1-hexene) obtained with Cat-C, D and E at 140 °C.