Figures & data
Figure 1. Synthetic route of P1–P4. Reagents and conditions: (i) In case of the Suzuki cross coupling reaction: Pd(PPh3)4/potassium carbonate/Aliquat 336©/water/toluene/120 °C; in case of the Stille cross coupling reaction: Pd(PPh3)4/toluene/100 °C.
Figure 2. Normalized absorption spectra of the prepared copolymers P1–P4 and small molecule SM-Bi-DPP (a) in chloroform and (b) as thin film.
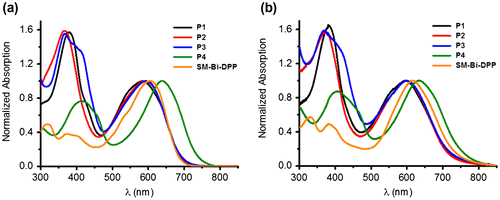
Figure 3. Cyclic voltammograms of the prepared copolymers and the small molecule: (a) Reduction and (b) oxidation.
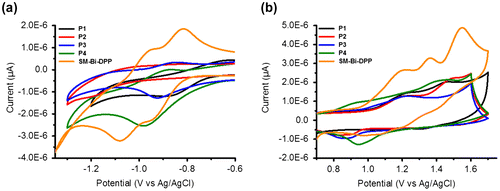
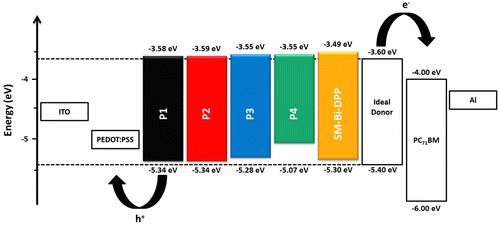
Figure 4. Illustration of the energy level diagrams of the prepared copolymers as well as of the small molecule.
Scheme 1. Schematic representation of the synthesis of Bi-DPP. Reagents and conditions: (i) Ethyl acetate/lithium diisopropylamide/tetrahydrofuran/−78 °C; (ii) pyridinium chlorochromate/celite/dichloromethane/25 °C; (iii) ethyl 2-chloroacetate/potassium carbonate/sodium iodide/acetone/55 °C; (iv) ammonium acetate/acetic acid/120 °C; (v) thiophene-2-carbonitrile/Na-t-OC5H12/80 °C; (vi) 2-ethylhexyl iodide/potassium carbonate/dimethyl sulfoxide/100 °C; and (vii) bromine/chloroform/25 °C.
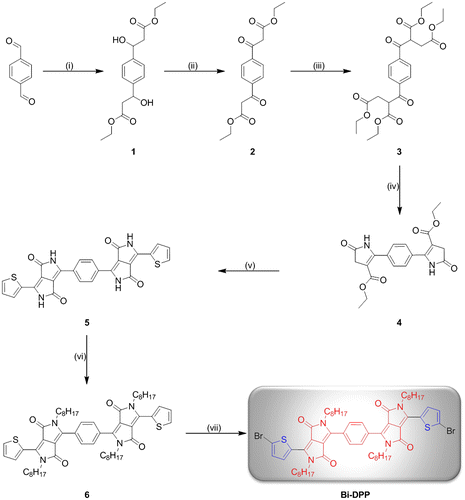
Table 1. Summarized molar mass and Ð values of the prepared copolymers.
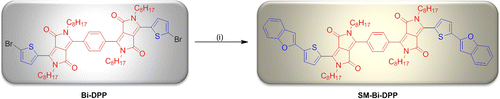
Scheme 2. Schematic representation of the synthesis of SM-Bi-DPP. Reagents and conditions: (i) Benzofuran-2-ylboronic acid/potassium carbonate/Pd2(dba)3/((t-butyl)3PH)BF4/tetrahydrofuran/water/25 °C.