Figures & data
Table 1. Formulation sheet of NaAlg/AA hydrogels.
Table 2. Dynamic (q) and equilibrium swelling (Eq) ratios of NaAlg/AA hydrogels.
Table 3. Amount of diclofenac potassium loaded and released (%) in different formulation of NaAlg/AA hydrogels.
Figure 3. Dynamic swelling ratio (q) of NaAlg/AA hydrogels with different concentrations of AA (26, 32 and 38 g) using EGDMA as crosslinking agent (0.4 wt%) in solution of different pH in 0.05 M USP phosphate buffer.
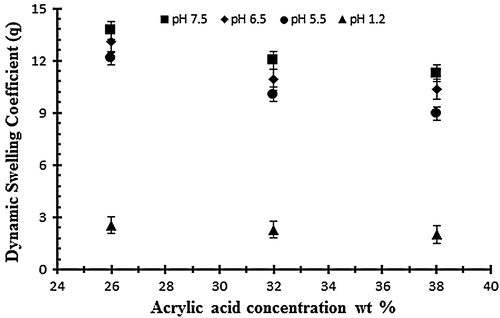
Figure 4. Release of diclofenac potassium from NaAlg/AA hydrogels using different concentrations of AA (26, 32 and 38 g) at various pH values in 0.05 M USP phosphate buffer.
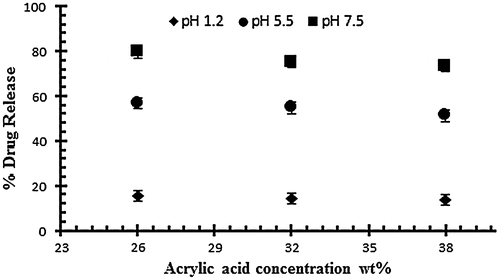
Figure 5. Dynamic swelling ratio (q) of NaAlg/AA hydrogels with different concentrations of NaAlg (1.5, 2 and 2.5 g) using EGDMA as crosslinking agent (0.4 wt%) in solution of different pH in 0.05 M USP phosphate buffer.
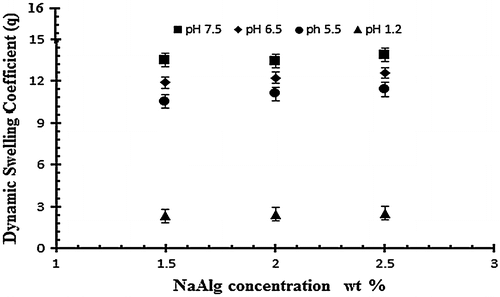
Figure 6. Dynamic swelling ratio (q) of NaAlg/AA hydrogels with different concentrations of EGDMA (0.3, 0.5 and 0.6 wt%) as crosslinking agent in solution of different pH in 0.05 M USP phosphate buffer.
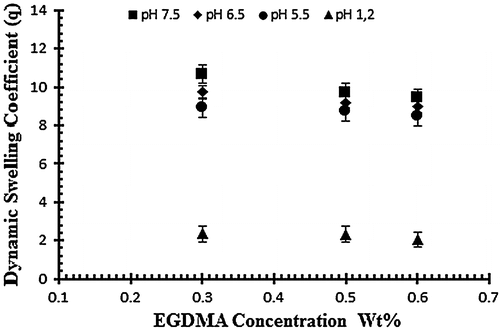
Figure 7. Release of diclofenac potassium from NaAlg/AA hydrogels using different concentrations of EGDMA as crosslinking agent (0.3, 0.5 and 0.6 various pH values in 0.05 M USP phosphate buffer.
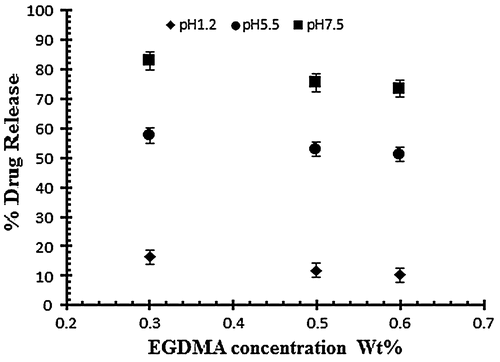
Table 4. Flory–Huggins network parameter of NaAlg/AA hydrogels.
Table 5. Effect of reaction variables on gel fraction and porosity.
Figure 8. Effect of variable concentration on gel fraction (a) NaAlg concentration (b) Acrylic acid concentration and (c) EGDMA concentration.
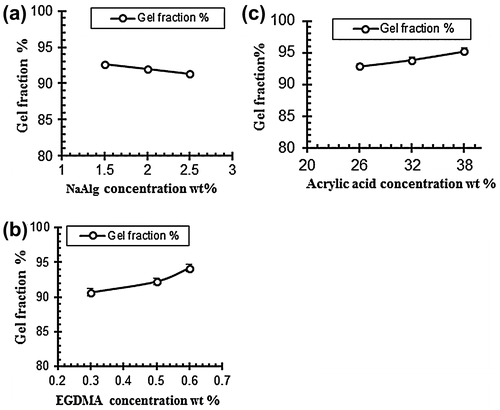
Figure 9. Effect of variable concentrations on porosity % (a) NaAlg concentration (b) Acrylic acid concentration and (c) EGDMA concentration.
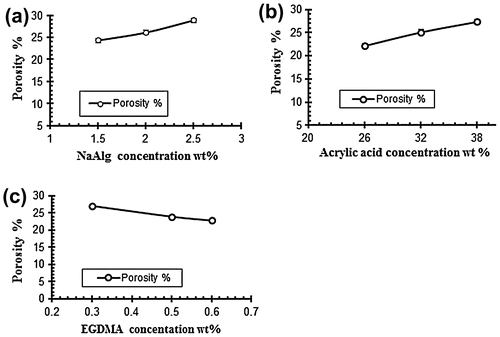
Table 6. Effect of different concentration of acrylic acid on drug release kinetics of NaAlg/AA hydrogels in solutions of different pH values using EGDMA as crosslinking agent (0.4% of AA).
Table 7. Effect of degree of crosslinking on drug release kinetics of NaAlg/AA hydrogels in solutions of different pH values.
Table 8. Effect of different concentration of acrylic acid on drug release mechanism of NaAlg/AA hydrogels in solutions of different pH values using EGDMA as crosslinking agent (0.4% of acrylic acid).
Table 9. Effect of degree of crosslinking on drug release mechanism of NaAlg/AA hydrogels in solutions of different pH values.
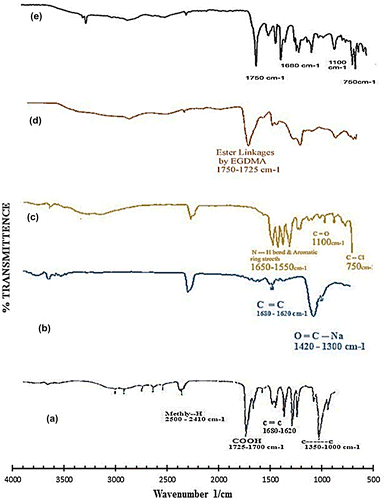
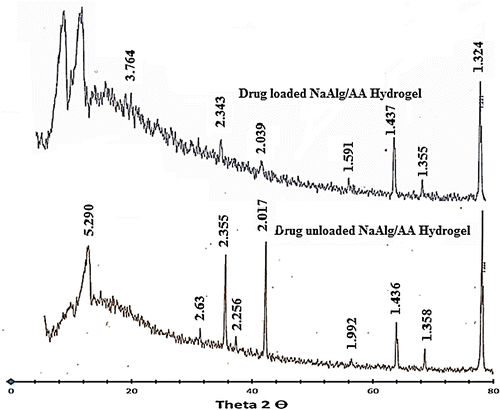
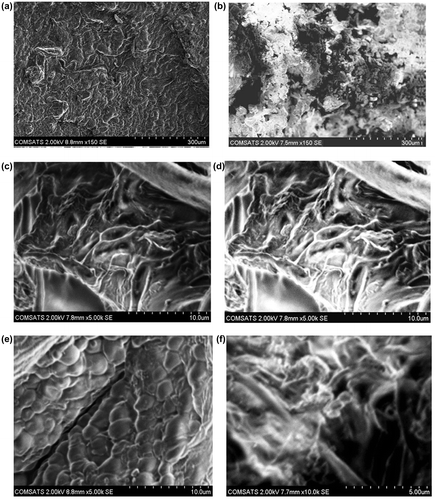
Figure 10. FTIR spectra of (a) acrylic acid (b) sodium alginate (c) diclofenac potassium (d) unloaded hydrogel and (e) loaded hydrogel.