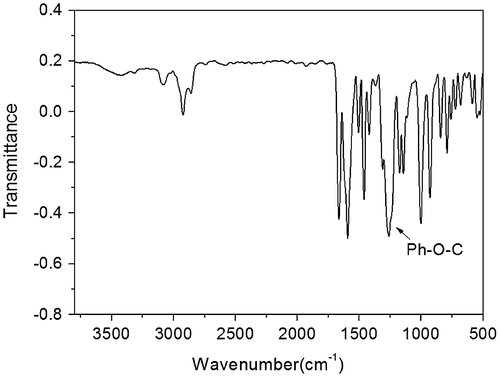
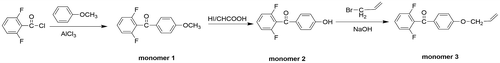
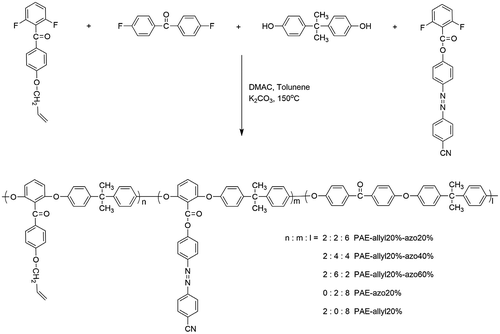
Figures & data
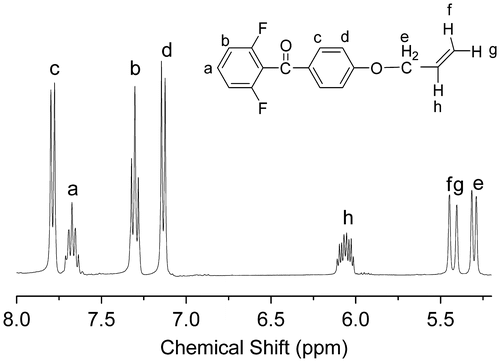
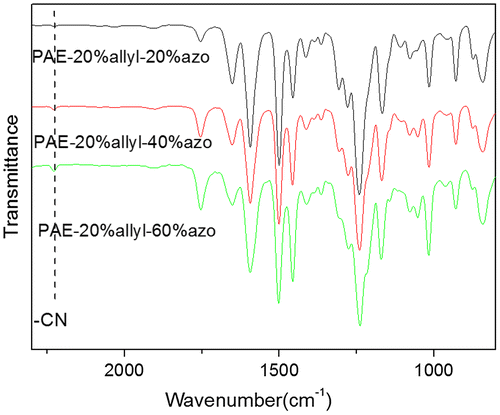
Figure 5. 1H NMR spectra of the polymers:(A) PAE-allyl20%; (B) PAE-azo20%; (C) PAE-allyl20%-azo20; (D)PAE-allyl20%-azo40%; (E) PAE-allyl20%-azo60%.
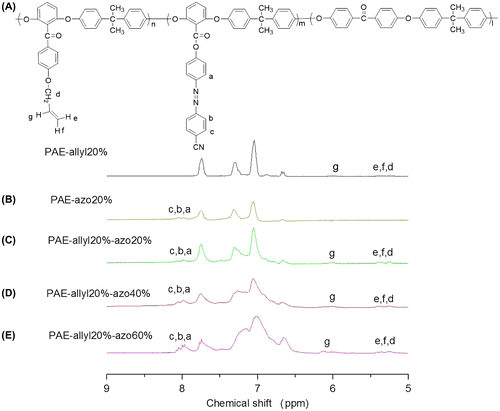
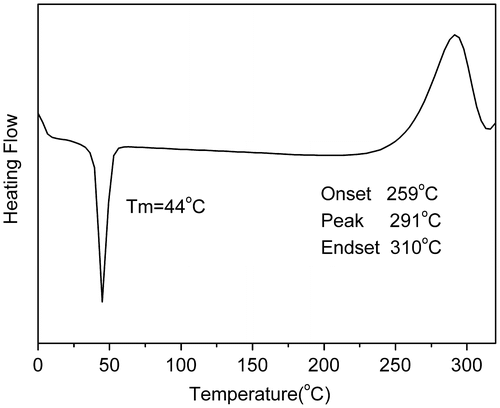
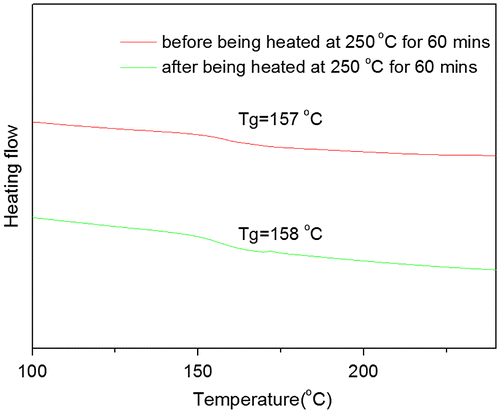
Figure 8. DSC curves of PAE-allyl20%-azo20% heated at 250 °C for different time: 0, 20, 40 and 60 min.
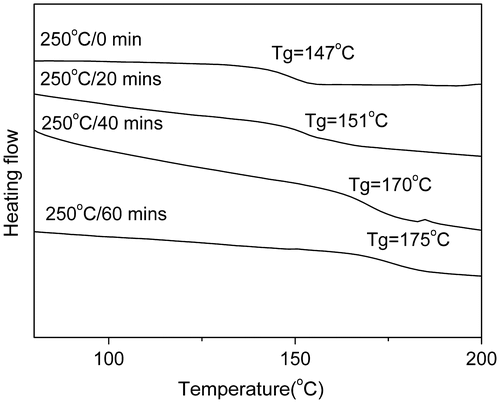
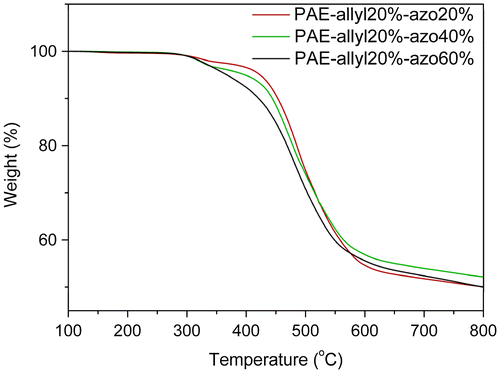
Figure 10. TGA curves of PAE-allyl20%-azo20% after being heated at 250 °C for different time (20, 40 and 60 min).
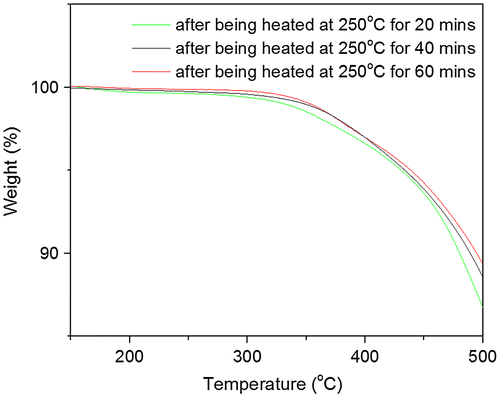
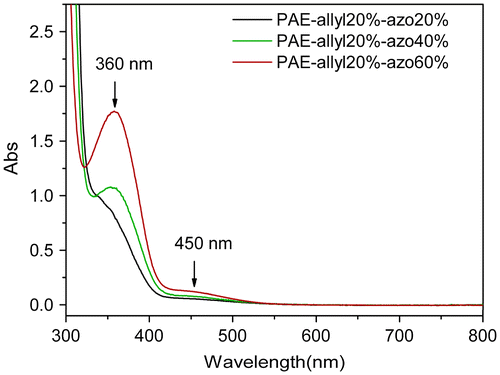
Figure 11. Typical behaviour of the photoinduced birefringence of PAE-allyl20%, PAE-allyl20%-azo20%, PAE-allyl20%-azo40% and PAE-allyl20%-azo60%: at point A, the writing laser was turned on; at point B, the writing laser was turned off; at point C, the circularly polarized light was turned on.
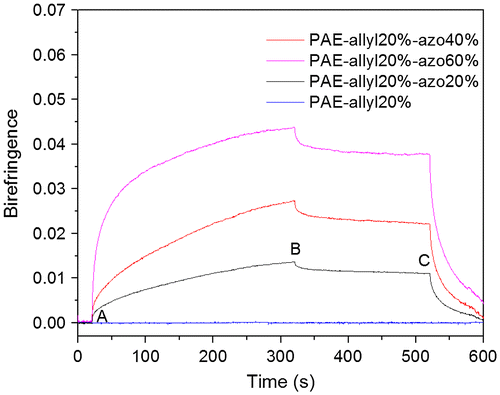
Figure 12. Typical behaviour of the photoinduced birefringence of PAE-allyl20%-azo20% before and after crosslinking.
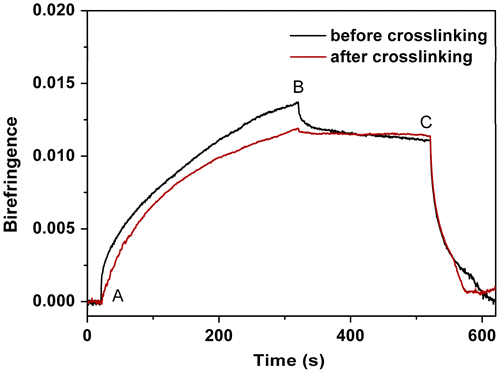
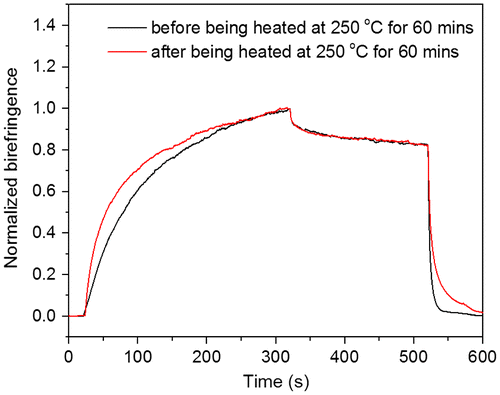
Figure 13. Normalized photoinduced birefringence of PAE-azo20% before and after being heated at 250 °C for 60 min.