Figures & data
Table 1. Summary of kinetic characteristics for homopolymarization of monomer M1. Polymerizations in MeOH and dioxane initiated by AIBN.
Figure 1. Changes of the part of ATR-FTIR spectra of M1 during homopolymerization. 0.25 mol/L M1; 0.025 mol/L AIBN; MeOH; 60°C.
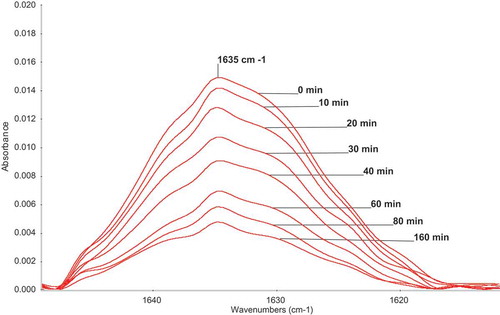
Figure 3. Dependence of M1 initial polymerization rates on starting monomer concentrations in MeOH at 60°C; [AIBN] = 0.05 mol/L.
![Figure 3. Dependence of M1 initial polymerization rates on starting monomer concentrations in MeOH at 60°C; [AIBN] = 0.05 mol/L.](/cms/asset/3216f115-b903-4ac8-9f17-02c099dda072/tdmp_a_1582216_f0003_b.gif)
Figure 4. M1 initial polymerization rates in dependence on AIBN initiator concentrations (60°C; monomer concentration 0.5 mol/L; MeOH).
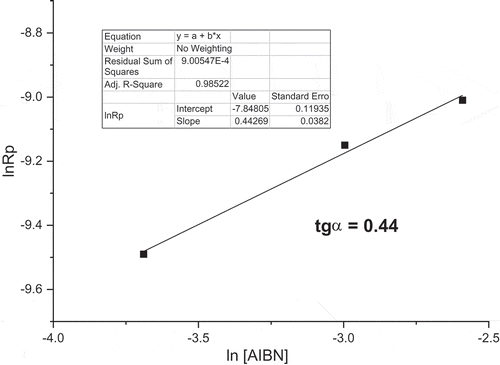
Figure 5. Monomer conversion of M1 during homopolymerization in dioxane. [AIBN] = 0,05 mol/L, T = 60°C.
![Figure 5. Monomer conversion of M1 during homopolymerization in dioxane. [AIBN] = 0,05 mol/L, T = 60°C.](/cms/asset/9cdb5427-aaa0-4067-9984-1c6a2ed332ef/tdmp_a_1582216_f0005_b.gif)
Figure 6. M1 initial polymerization rates in dependence on monomer concentration in the Arrhenius coordinates ([AIBN] = 0.05 mol/L, 60°C, dioxane).
![Figure 6. M1 initial polymerization rates in dependence on monomer concentration in the Arrhenius coordinates ([AIBN] = 0.05 mol/L, 60°C, dioxane).](/cms/asset/58047f04-43ff-4274-acca-a4547bfe0a05/tdmp_a_1582216_f0006_b.gif)
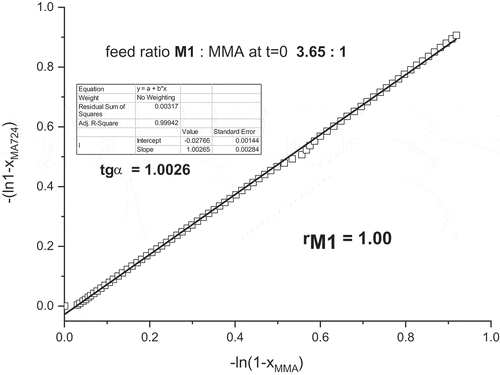
Figure 7. Details of the 1H-NMR spectra of the copolymerization of M1 with MMA at various time in deuterated methanol, T = 55°C. Molar ratio M1:MMA = 3.65:1 total monomers concentration = 0.5 mol/L, [AIBN] = 0.025 mol/L, CDCl3.
![Figure 7. Details of the 1H-NMR spectra of the copolymerization of M1 with MMA at various time in deuterated methanol, T = 55°C. Molar ratio M1:MMA = 3.65:1 total monomers concentration = 0.5 mol/L, [AIBN] = 0.025 mol/L, CDCl3.](/cms/asset/09398062-c769-4572-a3b4-ab8af6a1edcb/tdmp_a_1582216_f0007_b.gif)
Figure 8. (a) Monomer conversions of M1, MMA and total conversion during the copolymerization of mixture M1: MMA = 3.65:1. (b) comonomer ratio M1: MMA in feed versus time.
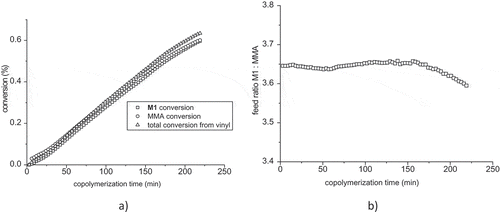
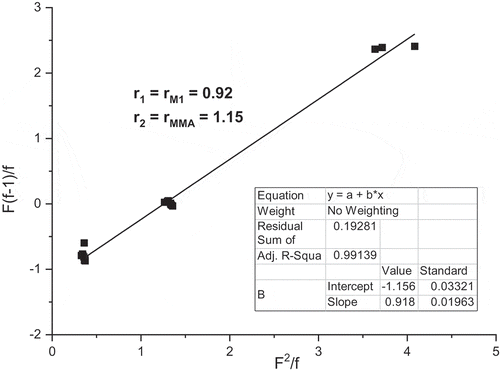
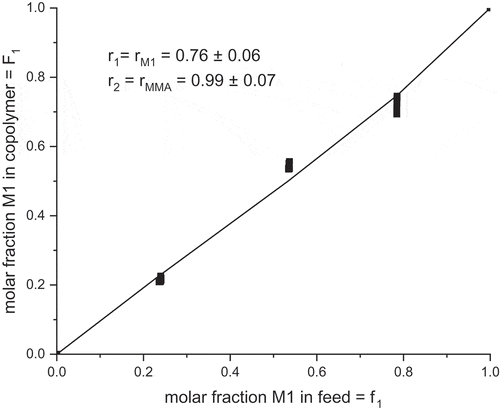
Table 2. Reactivity ratio of M1 and MMA determined by different methods.

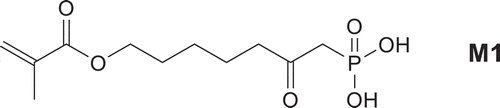
![Figure 2. Monomer conversion of M1 during homopolymerization. [AIBN] = 0,05 mol/L, T = 60°C, MeOH.](/cms/asset/e66042f3-9258-4ceb-a777-a135bdbac66e/tdmp_a_1582216_f0002_b.gif)