Figures & data
Table 1. Silver nanoparticles prepared via chemical reduction by the green tea extract
Figure 1. A green preparation process of the hydrogels. Due to the flavonoids and catechins in green tea, the green tea extract was used as the natural reducing agent to synthesize silver nanoparticles (AgNPs). Then, the obtained AgNPs were incorporated into polyvinyl alcohol/sodium alginate (PVA/SA) network to fabricate the hydrogels for antibacterial applications
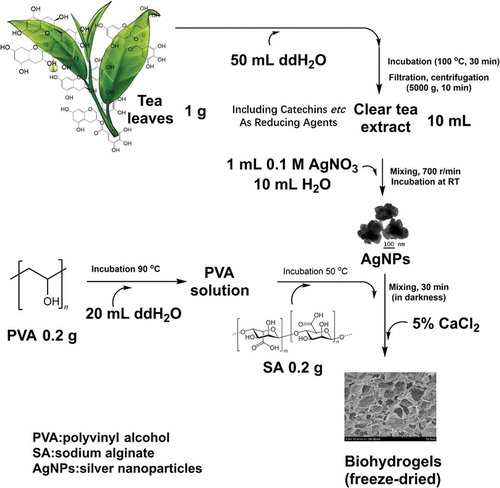
Figure 2. TEM images of AgNPs prepared by the reduction of AgNO3 with reductive compounds from tea leaves. The AgNPs had a distinct uniform interparticle separation. Images of four different preparations T-AgNPs-1 to 4 (A-D). A local area of T-AgNPs-3 was observed at two different magnifications (E, F)
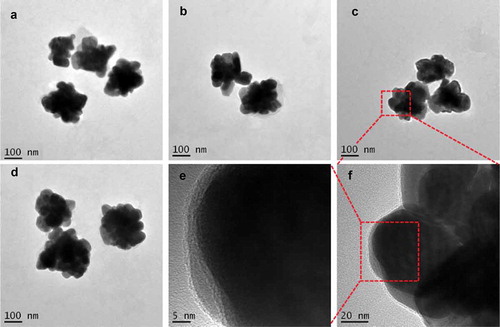
Figure 3. DLS analysis of the AgNPs dispersed in distilled water. The AgNPs had different average sizes, however, they could form a monodisperse suspension. T-AgNPs-1 to 4 was corresponding to A to D
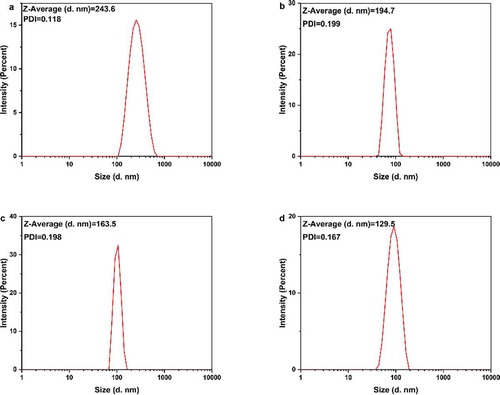
Figure 4. Zeta potential analysis of prepared silver nanoparticles dispersed in Millipore water. It indicated that T-AgNPs-1 and 2 had moderate stability, while T-AgNPs-3 and 4 could only form delicate dispersion
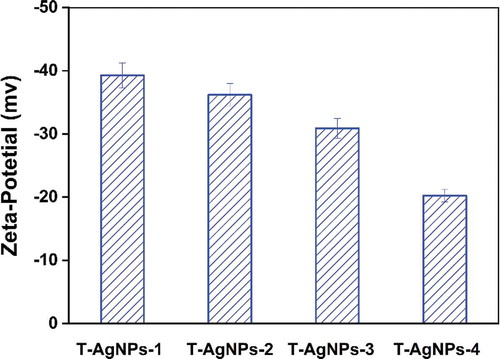
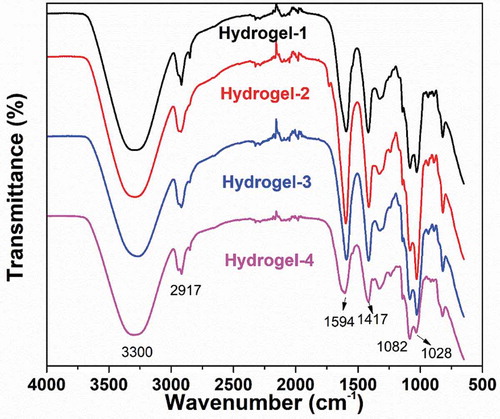
Figure 5. FT-IR spectra of polyvinyl alcohol, sodium alginate, and PVA/SA/AgNPs nanocomposite hydrogel (Hydrogel-1)
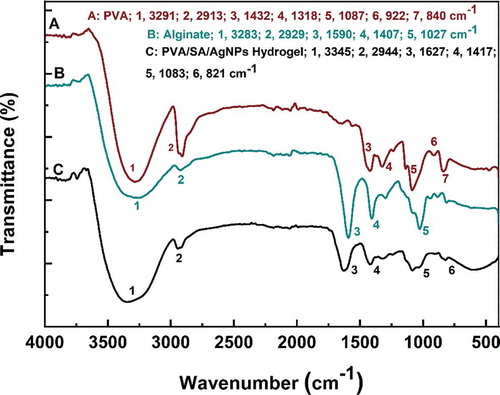
Figure 7. SEM images of prepared freeze-dried PVA/SA/AgNPs hydrogel. Hydrogels-1 was made from T-AgNPs-1 (A, B), and Hydrogels-2 from T-AgNPs-2 (C, D), Hydrogels-3 from T-AgNPs-3 (E, F), Hydrogels-4 from T-AgNPs-4 (G, H)
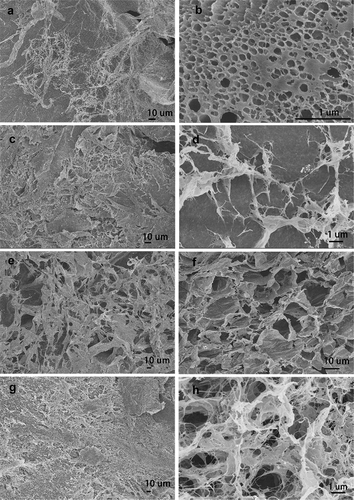
Figure 8. EDX analysis of prepared freeze-dried PVA/SA/AgNPs hydrogel. A: Hydrogel-1; B: Hydrogel-2; C: Hydrogel-3; D: Hydrogel-4
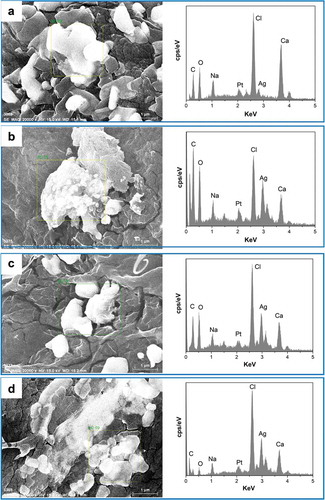
Figure 9. Swelling ratios of PVA/SA/AgNPs nanocomposite hydrogels. The hydrogels exhibited excellent swelling behavior. The hydrogel-1 and Hydrogels-4 had an equilibrium swelling ratio of about 900%, while hydrogel-2 and hydrogel-3 had a ratio of 500%. However, all these hydrogels could reach equilibrium in 20 min
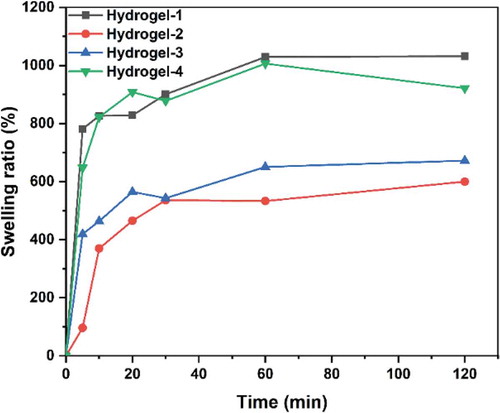
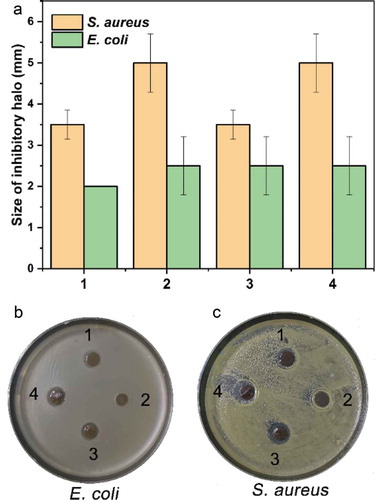
Figure 10. The antimicrobial activity of the hydrogels. The hydrogels had a different growth inhibitory effect on the growth of Gram-positive (E. coli) and Gram-negative (S. aureus) bacteria, which was indicated by the size of the inhibitory halo (A) and photos (B and C). By comparison, the hydrogels had a relatively more substantial inhibitory effect on the Gram-positive bacterium than on the negative