Figures & data
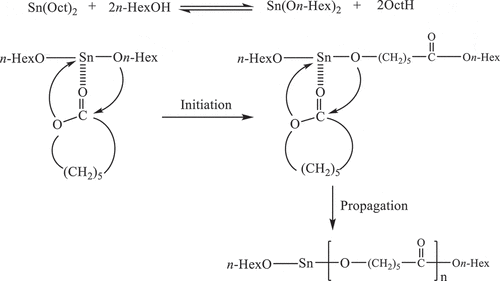
Figure 1. Non-isothermal DSC curves for the ROP of ε-CL initiated by SnOct2/n-HexOH (1:2): (a) 1.0, (b) 1.5, (c) 2.0 mol% at heating rates of 5, 10, 15 and 20°C/min and (d) non-isothermal DSC curve for the ROP of ε-CL initiated by 1.0, 1.5 and 2.0 mol% Sn(Oct2)/n-HexOH (1:2) at a heating rate of 10°C/min.
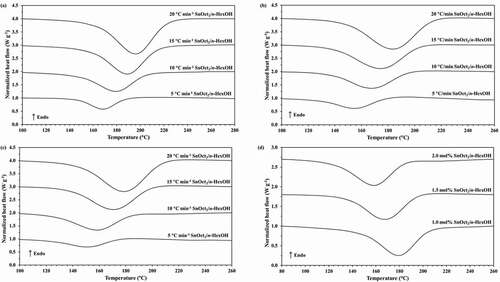
Figure 2. Plots of monomer conversion against temperature for the ROP of ε-CL initiated by (a) 1.0, (b) 1.5 and (c) 2.0 mol% of Sn(Oct)2/n-HexOH (1:2) at heating rates of 5, 10, 15, and 20°C min−1.
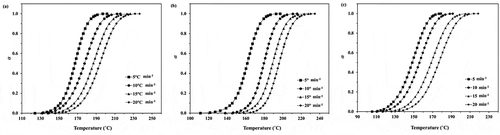
Figure 3. Plots of polymerization rate against temperature for the ROP of ε-CL initiated by (a) 1.0, (b) 1.5 and (c) 2.0 mol% of Sn(Oct)2/n-HexOH (1:2) at heating rates of 5, 10, 15, and 20°C min−1.
Figure 4. The KAS plots for the ROP of ε-CL initiated by (a) 1.0, (b) 1.5 and (c) 2.0 mol% of Sn(Oct)2/n-HexOH (1:2).
Figure 5. Plots of Ea against monomer conversion obtained from the ROP of ε-CL initiated by 1.0, 1.5 and 2.0 mol% of Sn(Oct)2/n-HexOH (1:2): (a) Friedman and (b) KAS isoconversional method.
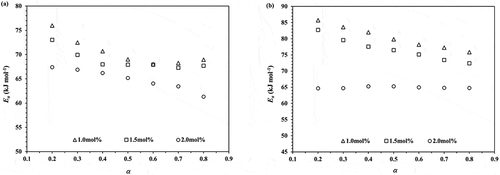
Table 1. Ideal reaction mechanism and kinetics model for solid state reaction [Citation12,Citation18,Citation26]
Table 2. Compensation parameters for f(α) = m(1-α)m[-ln(1-α)]m−1/m (m = 1.5 and 2.0) for the ROP of ε-CL with Sn(Oct)2/n-HexOH (1:2) at different heating rates
Figure 6. Plots of f(α) against α for the reaction models shown in (line) and the experimental plots of f(α) against α for the ROP of ε-CL initiated by 1.0, 1.5 and 2.0 mol% of Sn(Oct)2/n-HexOH (1:2) at a heating rate of 5 °C min−1 (dot).
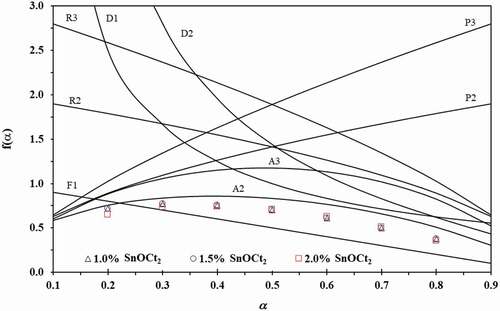
Table 3. Number average molecular weight (), weight average molecular weight (
), polydispersity index (PDI) and %yield of PCLs from bulk polymerization of ε-CL initiated by Sn(Oct)2/n-HexOH at 140, 160 and 180 °C for 1 h