Figures & data
Table 1. The data of crystal for the complex 1.
Figure 1. The coordination surrounding view of Cu(II) ions in the complex 1 (symmetry codes: (a) 0.5 + z, 1 – y, 2.5 – x; (b) z, y, 1 + x; (c) 1 – z, 1.5 – y, 0.5 + x)
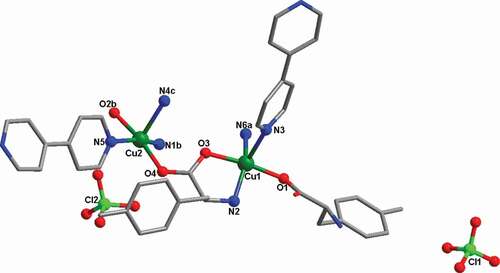
Figure 2. (a) The one-dimensional chain motif established via Cu(II) ions and the ligand of L. (b) The complex 1’s 3D skeleton with the free perchlorate anions filled in the channels
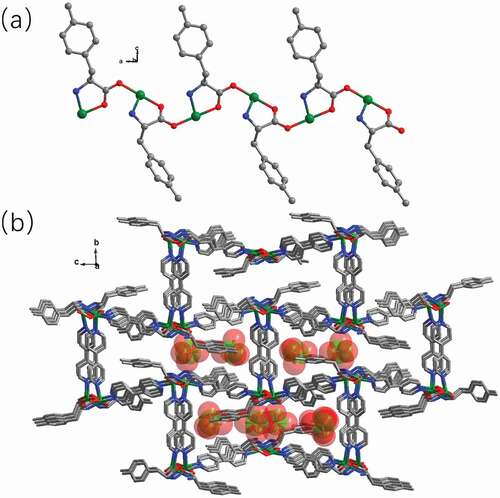
Figure 4. (a) The complex 1’s ultraviolet-visible diffuse reflectance spectrum. (b) The complex 1’s Kubelka–Munk transformed diffuse reflectance spectrum
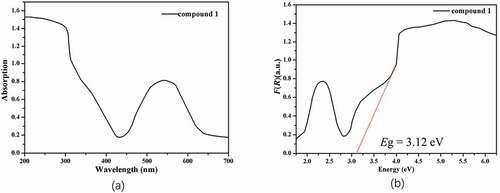
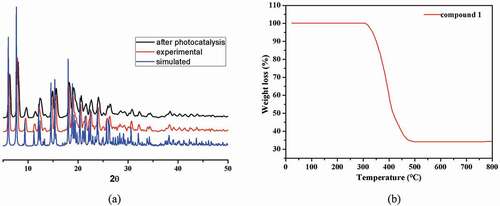
Figure 5. (a) The curve of irradiation time versus MB concentration in the existence of 1. (b) The photocatalytic decomposition for the solution of MB under the irradiation of ultraviolet light by the complex 1 and the control investigation without utilizing the catalyst. (c) The linear logarithmic plot as the function of time of ultraviolet light irradiation in the existence of 1. (d) Cycling three runs of the photocatalytic degradation of MB by 1.
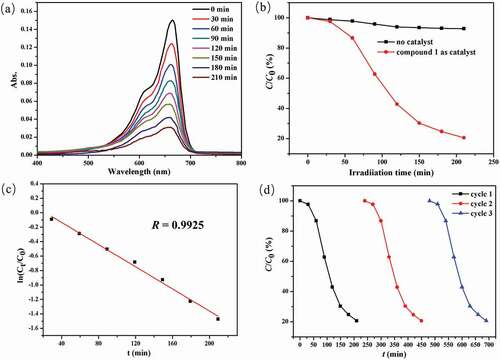
Figure 6. Significant reduction of the blood glucose levels in vivo after the compound treatment. The diabetes animal model was constructed and then the treatment was carried out with the compound at 1, 2, and 5 mg/kg concentration. The blood glucose levels in vivo were measured with blood glucose meter
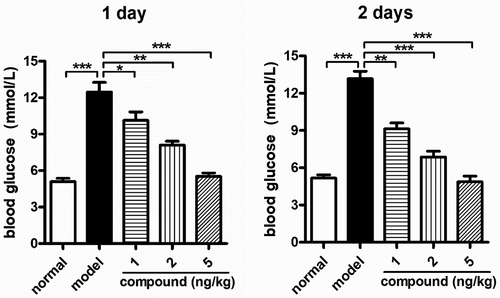
Figure 7. Remarkably downregulated VDAC1 relative expression levels in β-cells. The animal model of diabetes was created and then the treatment was performed with the compound at 1 , 2, and 5 mg/kg concentration. The real-time RT-PCR was implemented, and the VDAC1 relative expression levels in β-cells were accomplished
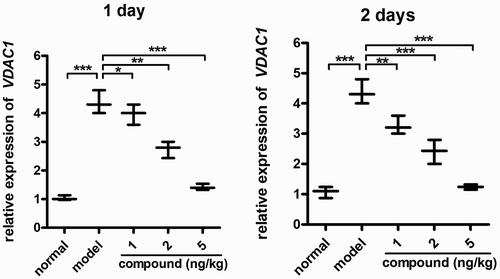
Figure 8. No cytotoxicity of the compound on β-cells. The β-cells in the logical growth phage were collected and treated with serial different dilutions of the compound for 48 h incubation. The viability of the new compound was evaluated with CCK-8 assay
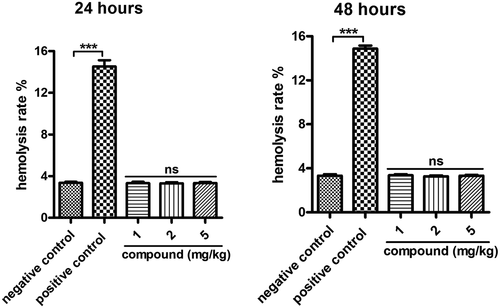
Figure 9. No hemolysis toxicity of the compound on β-cells. Blood used in the hemolysis experiment was taken from adult male New Zealand white rabbits and incubated with serial different dilutions of the new compound for 48 h incubation. The degree of red blood cell lysis and hemoglobin release caused by the compound was evaluated