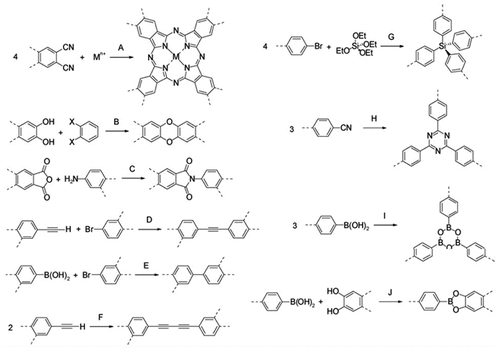
Figures & data
Figure 1. Aryl hydrazine by co-thermal reaction with naphthol in the presence of sodium bisulfite and cyclized under acidic conditions to produce tetrahydrocarbazole and catalytically dehydrogenated to produce carbazole.
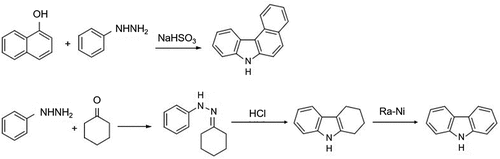
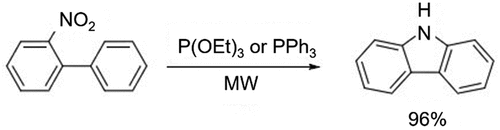
Figure 2. Oxidative dehydrogenation of 2-aminobiphenyl or deamination of 2,2’-diaminobipheny for the preparation of carbazole.
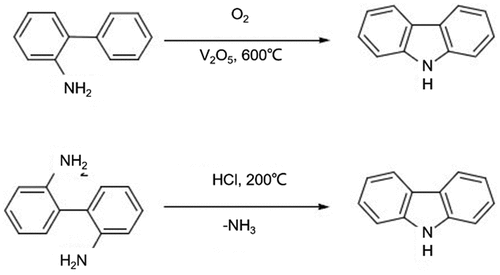
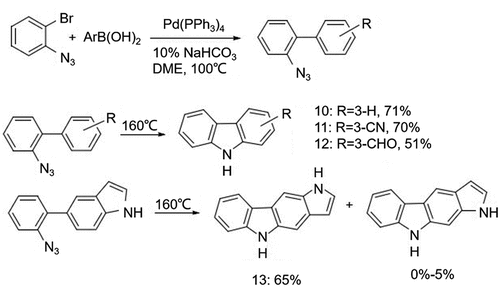
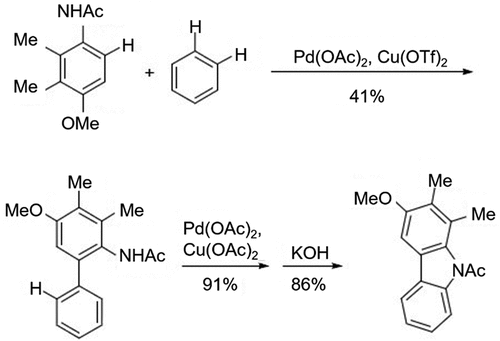
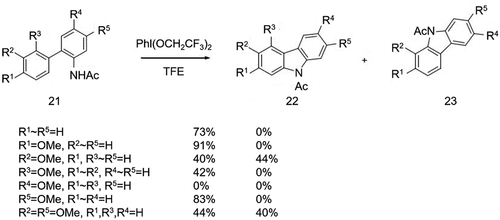
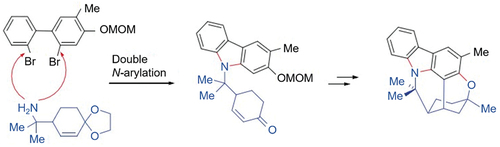
Figure 3. (a) Suzuki-Miyaura coupling reaction to synthesize biphenyl intermediates with different substituents and (b) cyclization using different nitrogen-containing groups and catalysts.
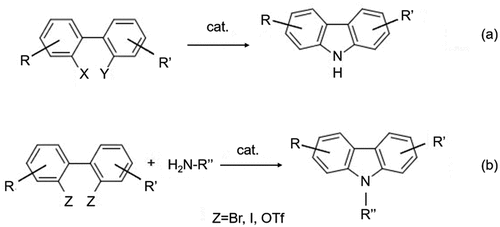
Figure 4. Synthesis of carbazoles and carbazole derivatives using disubstituted nitrobiphenyls in presence of MoO2Cl2(dmf)2 and PPh3.
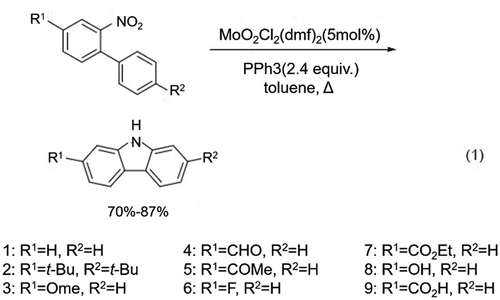
Figure 5. Siamenol synthesized process by cyclization of PPh3/dichlorobenzene system using nitrobiphenyl as the key intermediate.
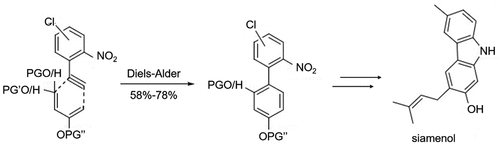
Figure 8. Carbazole derivatives synthesis using azidobiphenyl under the conditions of Rh2(O2CC3F7)4.
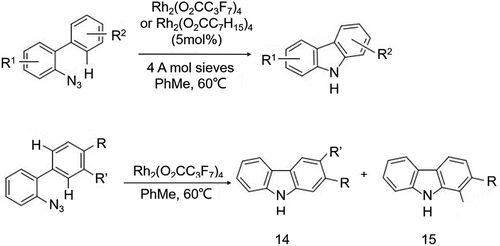
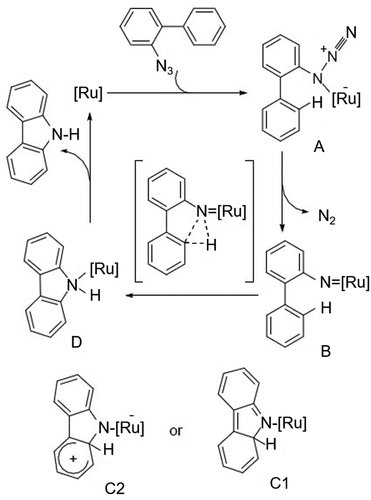
Figure 9. Ruthenium complexes series preparation [e.g., RuCl2-(PPh3)3, RuCl2(DMSO)4, RuCl3, RuO2, (NH4)2RuCl6] for the C-H amination reactions of organic azide compounds.
![Figure 9. Ruthenium complexes series preparation [e.g., RuCl2-(PPh3)3, RuCl2(DMSO)4, RuCl3, RuO2, (NH4)2RuCl6] for the C-H amination reactions of organic azide compounds.](/cms/asset/abe788af-ffe3-4a3e-a981-9883a2d7f5c6/tdmp_a_2194174_f0009_b.gif)
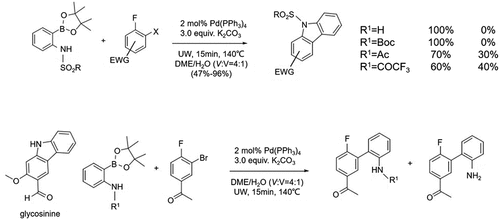
Figure 11. Direct synthesis of carbazoles from N-substituted aminophenylboronic ethers and o-dihalogenated benzenes.