Figures & data
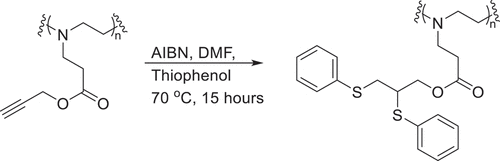
Scheme 1. Overall reaction scheme showing the partial hydrolysis of poly(2-oxazoline)s followed by the subsequent aza-Michael addition.
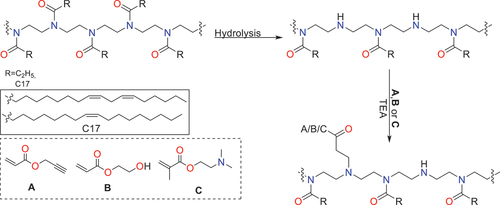
Table 1. Summary table for the conditions studied for the aza-Michael additions carried out for PEtOx (PE1-PE7) and PFAOx (PF1-PF4.).
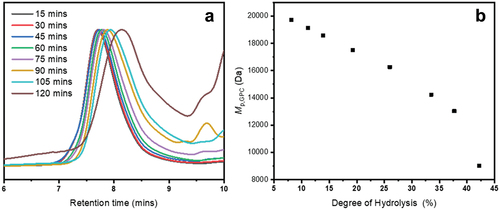
Figure 1. a) hydrolysis kinetics showing PFAOx hydrolysed in 8:2 THF/HClaq (black), PEtOx hydrolysed in 8:2 THF/HClaq (red), PEtOx hydrolysed in 6.3:1.3:2.4 1,4-butanediol/water/HClaq (blue), PEtOx hydrolysed in 8:2 THF/HClaq at 100 °C (green) and PEtOx hydrolysed in 8:2 water/HClaq (purple). b) corresponding first-order kinetic plot. All reactions were carried out with 35–37% HClaq such that [H+]=2.4 M and [A]=0.48 M. PFAOx hydrolysis was calculated according to EquationEquation 1(1)
(1) and all PEtOx hydrolysis rates were calculated according to Equation 12.
![Figure 1. a) hydrolysis kinetics showing PFAOx hydrolysed in 8:2 THF/HClaq (black), PEtOx hydrolysed in 8:2 THF/HClaq (red), PEtOx hydrolysed in 6.3:1.3:2.4 1,4-butanediol/water/HClaq (blue), PEtOx hydrolysed in 8:2 THF/HClaq at 100 °C (green) and PEtOx hydrolysed in 8:2 water/HClaq (purple). b) corresponding first-order kinetic plot. All reactions were carried out with 35–37% HClaq such that [H+]=2.4 M and [A]=0.48 M. PFAOx hydrolysis was calculated according to EquationEquation 1(1) DegreeofHydrolysis%=100IH,tf−IH,t0IP(1) and all PEtOx hydrolysis rates were calculated according to Equation 12.](/cms/asset/7e3ed19b-299b-47c4-a8e6-2a970c688186/tdmp_a_2267232_f0001_oc.jpg)
Supplemental Material
Download MS Word (301.9 KB)Data availability statement
FURTHER DATA WILL BE AVAILABLE UPON REQUEST.