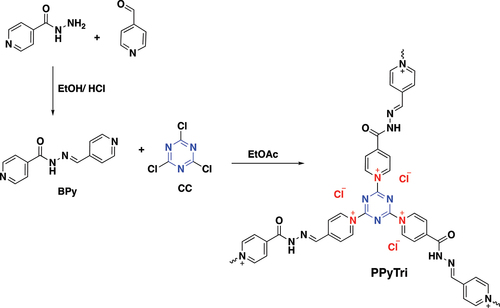
Figures & data
Scheme 2. Systematic illustration of nanocomposite fabrication for PPyTri/CNTs-5%, PPyTri/CNTs-2%, PPyTri/gnps-5%, and PPyTri/gnps-2%.
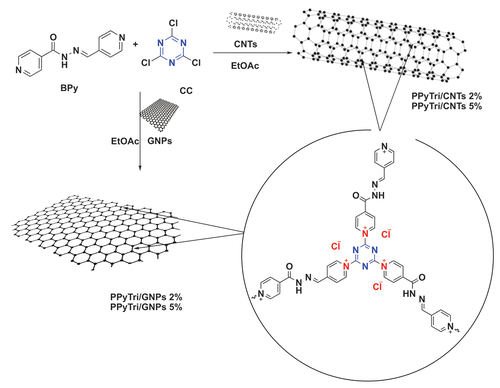
Figure 3. [Citation1]H NMR spectra for the monomer BPy and polymer PPyTri.
![Figure 3. [Citation1]H NMR spectra for the monomer BPy and polymer PPyTri.](/cms/asset/342e4350-d8cd-4287-acfa-c9a1123ba0cf/tdmp_a_2360746_f0003_oc.jpg)
Figure 4. [Citation13]C NMR1H NMR spectra for the monomer BPy and polymer PPyTri.
![Figure 4. [Citation13]C NMR1H NMR spectra for the monomer BPy and polymer PPyTri.](/cms/asset/be4d6a83-0d54-46c0-a019-931e66cb9aa5/tdmp_a_2360746_f0004_oc.jpg)
Figure 6. SEM images of the pure copolymer and its nanocomposites PPyTri, PPyTri/CNTs-2%, and PPyTri/gnps-2%.
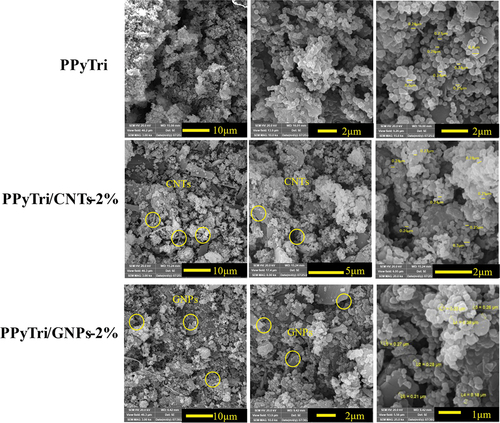
Figure 7. EDX elemental maps of the pure copolymer and its nanocomposites PPyTri, PPyTri/CNTs-2%, and PPyTri/GNPs-2%.
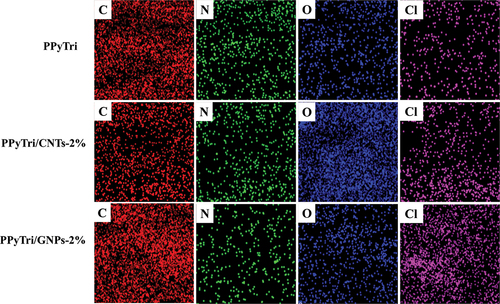
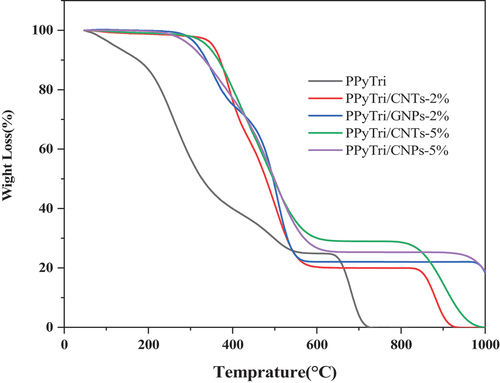
Table 1. Thermal behaviors of the polymer PPyTri and its nanocomposites.
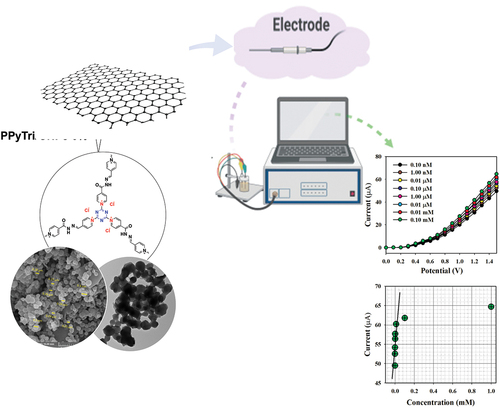
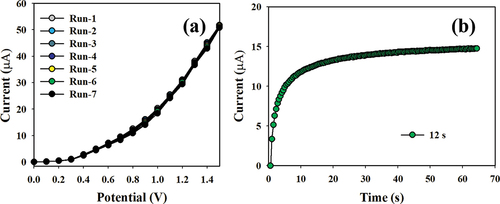
Figure 10. Classification of the sensor behaviors using the electrochemical (I-V) approach. (a) Selectivity estimation, (b) I-V responses based on variations in the Hg+2 ion concentration from low to high, and (c) calibration curve.
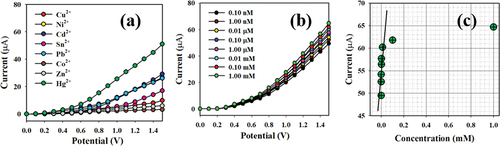
Figure 11. Control study executed at 0.1 µM Hg+2 solutions in a buffer medium with modified GCE containing PPyTri/GNPs or PPyTri/CNTs (2, 5%) nanostructure compositions.
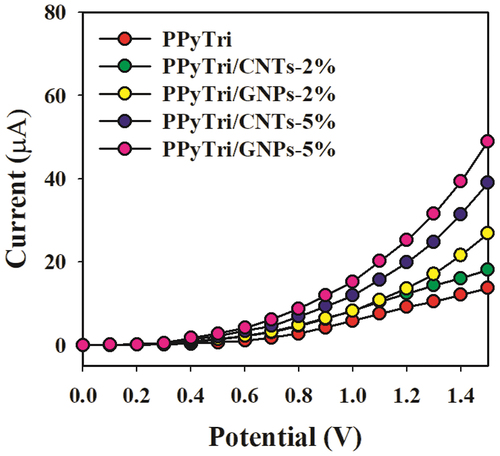
Table 2. Performance comparison of different electrochemical sensors for Hg+2 ion detection.
Table 3. Validation of PPyTri/GNPs-5% NCs fabricated sensor probe using real samples by recovery method.
Data availability statement
All the data has been illustrated in the manuscript text.