Figures & data
Fig. 2. The mixture preparation device consists of three saturators where the stream of the carrier gas is saturated with the desired vapour.
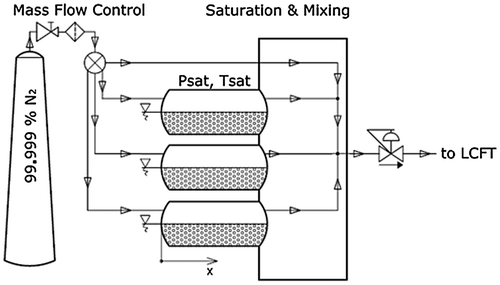
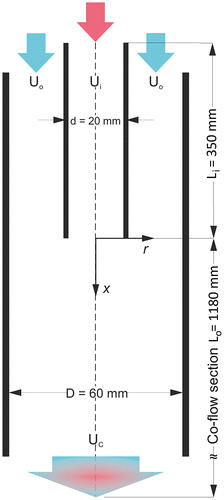
Fig. 3. A detailed depiction of the inner layout in the saturators used in the mixture preparation device.
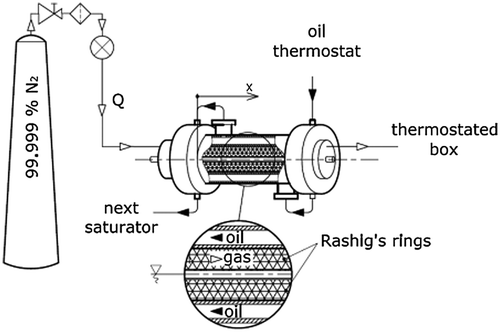
Table 1. Verified temperature range and maximal flow rate allowing stable and repeatable saturated vapour generation in the saturator.
Table 2. Parameters used in the experimental set up and the corresponding results, patm = 101.325 kPa.
Fig. 5. Postprocessing of measured concentrations, Tsat = 22.47 [°C], = 0.76 [l min–1],
= 0.25 [l min–1].
![Fig. 5. Postprocessing of measured concentrations, Tsat = 22.47 [°C], Q˙W = 0.76 [l min–1], Q˙SA = 0.25 [l min–1].](/cms/asset/166328df-404e-408e-b88d-9ba54b37db82/zelb_a_1446643_f0005_oc.gif)
Table 3. Deviations caused by different setting of limit concentration Nlimit, Tsat = 22.47 [°C], 
 = 0.76 [l min−1],
= 0.76 [l min−1], 
 = 0.25 [l min−1].
= 0.25 [l min−1].
Table 4. Results obtained by CFD simulations, patm = 101.325 kPa, coordinate of nucleation maximum on the axis of the co-flow section x = 0.53 cm.
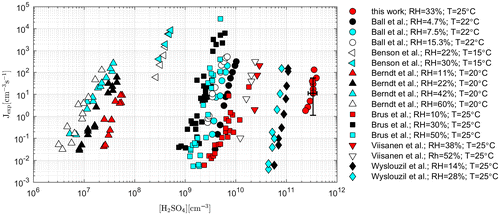
Fig. 6. Comparison of our results with data obtained by other authors using different types of experimental devices. The dependence of the experimental nucleation rate on the concentration of sulphuric acid in the chamber during the constant value of Rh. Uncertainties of experimental data obtained by LCFT are marked.