Figures & data
Table 1. Participant demographics and characteristics.
Figure 1. SF-36v2 domains, stratified by recency of last acute episode.
Note: The data represents the mean norm-based scores with the 95% CIs for each of the eight domains of the SF-36 and also for the two component summary measures. Data for each measurement are compared to the mean value for the US population that has been normalized to a mean score of 50, designated by the line.
PF=Physical Functioning; RP=Role Physical; BP=Bodily Pain; GH=General Health; V=Vitality; SF=Social Functioning; RE=Role Emotional; MH=Mental Health; PCS=Physical Component Score; and MCS=mental Component Score.
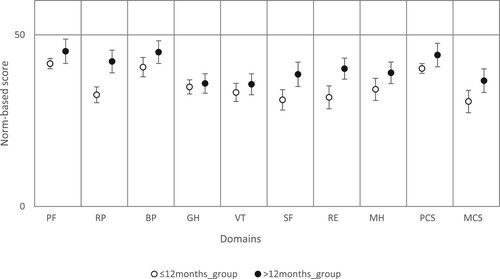
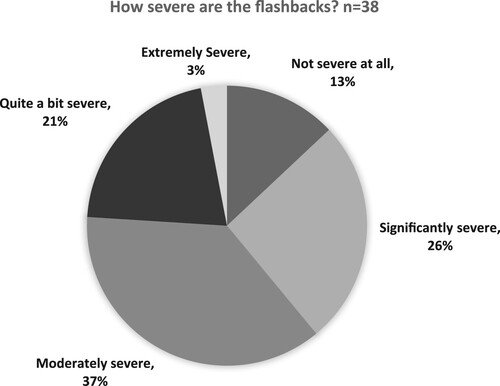
Table 2. Scores for PROMs.
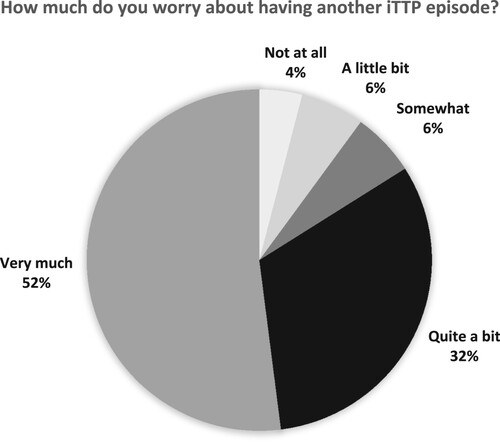
Table 3. Bespoke questions – activities of daily living, n = 50.
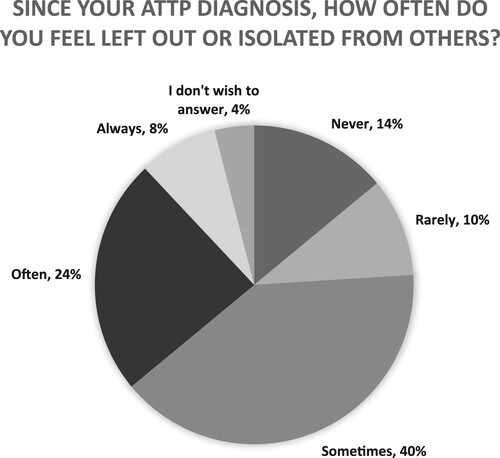
Table 4. Bespoke questions – fatigue interference, n = 50.
Supplemental Material
Download Zip (109.6 KB)Data availability statement
Qualified researchers may request access to patient-level data and related study documents including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient-level data will be anonymized and study documents will be redacted to protect the privacy of our trial participants. Further details on Sanofi’s data-sharing criteria, eligible studies, and process for requesting access can be found at: https://www.clinicalstudydatarequest.com/.