Figures & data
Figure 1. The onset of bleeding events according to aetiology and VWD treatment. The time from the last treatment infusion at spontaneous bleeding onset varied from 8 and 66 h during the pdVWF:pdFVIII period. The start of spontaneous bleeding occurred 8 h after the last treatment infusion during the FVIII-poor pdVWF period. Time from last treatment infusion at spontaneous bleeding onset varied from 5 and 58 h during the pdVWF:pdFVIII period and between 29 and 53 h during the FVIII-poor pdVWF period. Abbreviations: FVIII, factor VIII; pd, plasma-derived; VWD, von Willebrand disease; VWF, von Willebrand factor.
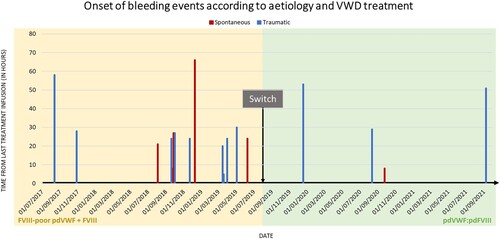
Figure 2 Pharmacokinetics analysis of pdVWF:pdFVIII and FVIII-poor pdVWF concentrates and the peak of thrombin generation (after infusion 42 IU/kg VWF) from platelet-poor plasma. Left axis: Peak of thrombin generation (concentration in nM), normal range (5-95th percentiles) 53–115 nM. Right axis: VWF or FVIII activity in percentage; VWF:Act% was measured using the VWF gain-of-function glycoprotein Ib assay (Innovance VWF Ac assay, Siemens), normal range for O blood type 124–49%. FVIII:C% was measured by the chromogenic assay (Biophen FVIII:C, Hyphen Biomed), normal range 70–150%. Abbreviations: FVIII, factor VIII; pd, plasma-derived; PPP; platelet-poor plasma; VWF, von Willebrand factor, Act%, activity percentage: C%, procoagulant activity percentage.
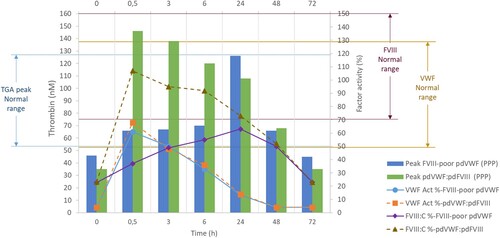
Table 1. Thrombin generation assay on PPP pharmacokinetics samples after pdVWF:pdFVIII and FVIII-poor pdVWF concentrates administration (after infusion 42 IU/kg VWF).
