Figures & data
Table 2. Inclusion parameters for targeted literature review.
Figure 1. Model structure and different states of patients. Abbreviations: PWH: Patients with hemophilia: Base state in which persons with hemophilia A (PWH) are entering and starting therapy; Joint disease state: in which PWH have extensive bleeds leading to joint disease (requiring major surgery). Model flow: Individuals diagnosed with hemophilia initially experience a bleeding incident, which can occur with or without joint involvement. When a bleeding incident transpires without joint involvement, patients may either revert to the base state or advance to the joint disease state. Conversely, when a bleeding incident involves joints, patients may either revert to the joint disease state or show improvement and return to the base state. Both the base state and the joint disease state are associated with the possibility of death. The minimum age for entering the model was 2 years for the pediatric population and 18 years for the adult population.
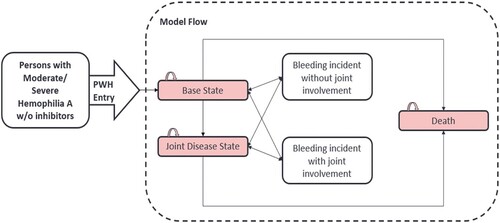
Figure 2. Study outcomes in terms of quality adjusted life years gained and total cost. Abbreviation: QALY: Quality adjusted life year.
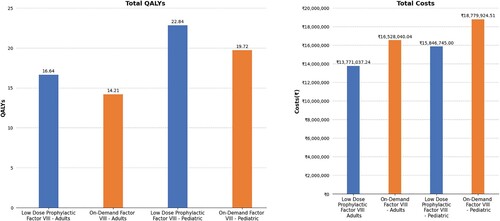
Table 1. Breakdown of cost distribution for adult and pediatric patients with hemophilia at ‘per patient level’.
Figure 3. One-way sensitivity analysis representation. Abbreviations: ICUR: Incremental cost-utility ratio; QALY: Quality adjusted life year.
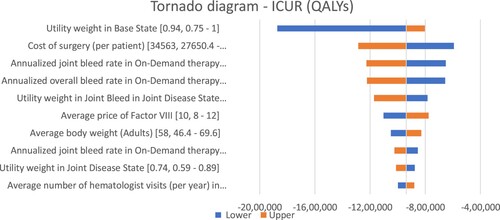
Figure 4. Probabilistic sensitivity analysis representing the cost-effectiveness cloud. Abbreviations: CE: Cost-effectiveness; QALY: Quality adjusted life year. The study analyzed certain probable factors that may affect the results of the study model. 1000 simulations were run to check the impact of these factors on the comparison between low-dose prophylactic factor and on-demand factor VIII therapy and the incremental scatter plot graph was generated. In majority of the simulations (99.8%), the low-dose prophylactic factor was better than on-demand factor VIII therapy which means that the low-dose prophylactic factor is not only less expensive but also more effective than on-demand factor VIII therapy. Hence, the factors tested had very little effect on the results of our study.
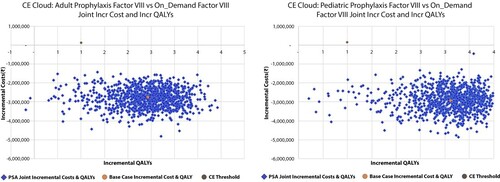
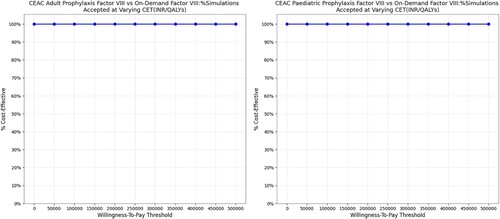
Figure 5. Probabilistic sensitivity analysis representing cost-effectiveness acceptability curve. Abbreviations: CEAC: Cost-effectiveness acceptability curve; INR: Indian Rupee; QALY: Quality adjusted life year. The PSA results were analyzed using the cost-effectiveness acceptability curve. The curve showed that Low-dose prophylactic factor therapy was below the cost-effectiveness threshold in all the simulations (100%) which indicates a cost-effective medication strategy. The prophylactic therapy was cost-effective for both adults and children, no matter what willingness-to-pay level was considered.
Data availability statement
The datasets, including the redacted study protocol, redacted statistical analysis plan, and individual participants data supporting the results reported in this article, will be made available within three months from initial request to researchers. The data will be provided after its de-identification, in compliance with applicable privacy laws, data protection and requirements for consent and anonymization
