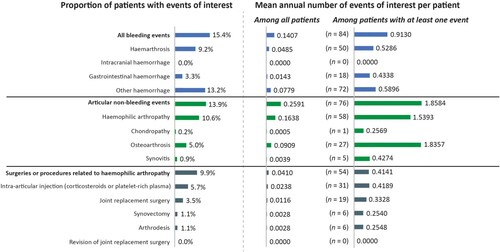
Figures & data
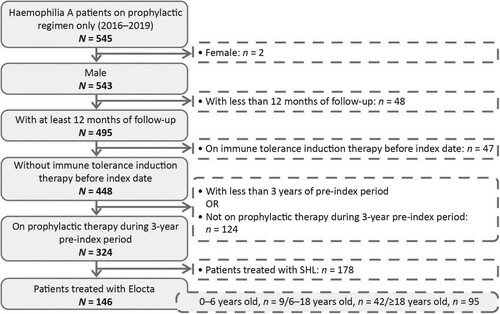
Figure 1. Flow chart depicting the selection of the overall population. FVIII, factor VIII; rFVIIa, recombinant factor VIIa; SNDS, Système National des Données de Santé.
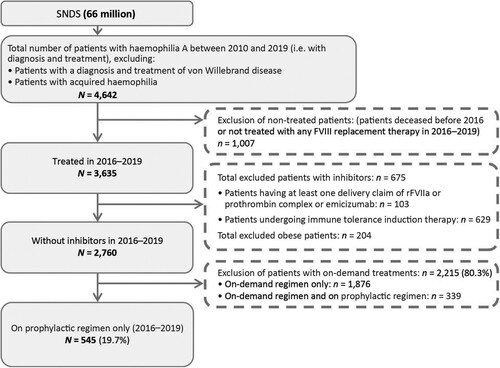
Table 1. Monthly consumption of FVIII replacement therapy per patient in IU/kg/month for the overall population.
SNDS suppl updated_15Dec23 clean.docx
Download MS Word (167.7 KB)Data availability
Sobi is committed to responsible and ethical sharing of data on participant level and summary data for medicines and indications approved by EMA and/or FDA, while protecting individual participant integrity and compliance with applicable legislation. Data access will be granted in response to qualified research requests. All requests are evaluated by a cross-functional panel of experts within Sobi and a decision on sharing will be based on the scientific merit and feasibility of the research proposal, maintenance of personal integrity and commitment to publication of the results. To request access to study data, a data sharing request form (available on www.sobi.com) should be sent to [email protected]. Further information on Sobi’s data sharing policy and process for requesting access can be found at: https://www.sobi.com/en/policies. The raw data were part of the SNDS and were available from the Health Data Hub under special authorization.