Figures & data
Figure 2. DNA and RNA analysis results of each family member. DNA (A-C) and RNA (D-F) results of the α2-globin gene indicated a homozygous and heterozygous variant of HBA2:c.175C > A (red arrow) in proband (A and D) and father (B and E), respectively, which was not identified in mother (C and F). Further, an equal ratio of normal and mutant alleles could be observed from cDNA sequencing result in the heterozygote father (E).
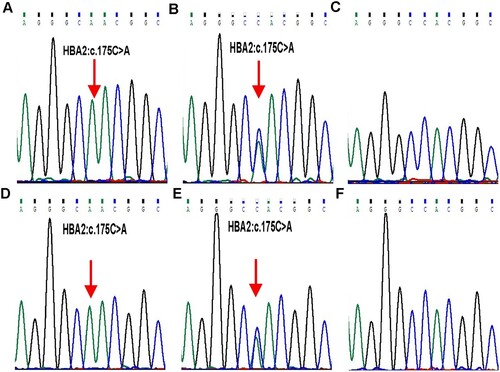
Figure 3. Stick models of α native (A) and (H58N) (B) distal pockets in adult human haemoglobin. The structure of Hb DG-Nancheng-O2 was generated from native HbA-O2 (PDB code 2DN1) by mutating the distal E7 residue His58 to Asn in the program Pymol. Red sphere: oxygen. The native His (E7) side chain forms a hydrogen bond with bound O2, in Hb DG-Nancheng-O2, the distal E7 residue Asn58 changes its side chain orientation out of the heme pocket and does not form a hydrogen bond with bound ligands. Nascent globin chains rapidly incorporate heme, which stabilizes their native folding into Hb subunits composed of seven or eight a helices named A–H, which fold together into a globular structure. The alteration by Asn58 possibly caused the inability of mutant α-chains bind to haeme, ultimately resulting in proteolytic degradation.
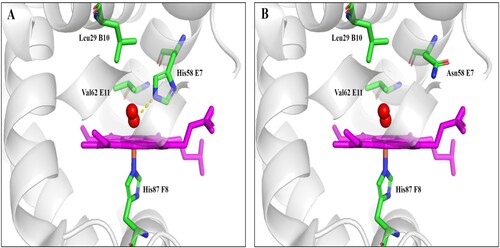
Table 1. Hematological data and α- and β- genotypes of the family members.
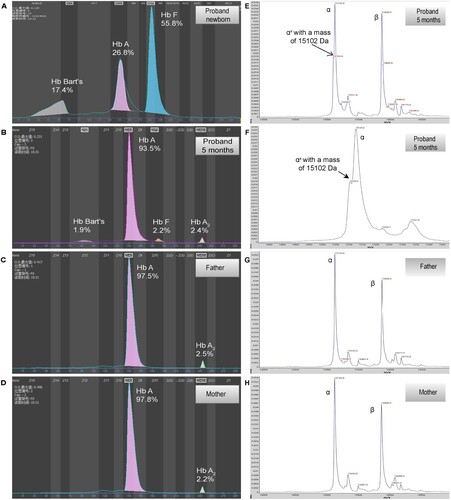
Figure 1. Hemoglobin patterns from capillary electrophoresis (CE) and MALDI-TOF. By CE, a Bart’s level of 17.5% and 1.9% was identified in proband at newborn (A) and 5 months (B), respectively. No abnormal peak was identified in father (C) and mother (D). By MALDI-TOF, a normal α chain at 15,127 m/z and an abnormal α chain (αX) at 15,103 m/z were identified in proband (E and F, the peak of abnormal α chain is zoomed at F), the abnormal α chain was not identified in father (G) and mother (H).