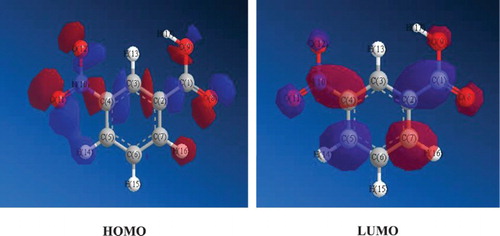
Figures & data
Figure 1. Variation of weight loss with time for the corrosion of mild steel in 0.1 M HCl containing various concentrations of NBA at 303, 313, 323 and 333 K.
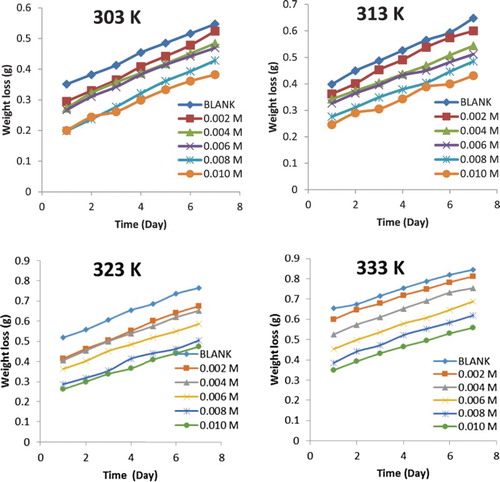
Figure 2. Variation of weight loss with time for the corrosion of aluminium in 0.1 M HCl containing various concentrations of NBA at 303, 313, 323 and 333 K.
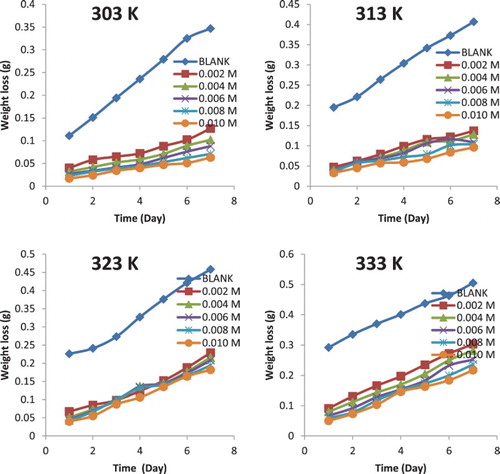
Table 1. Inhibition efficiency of NBA (from weight loss and thermometric measurements) and corrosion rate of mild steel and aluminium metals.
Table 2. Linear and potentiodynamic polarization parameters for the corrosion of aluminium and mild steel in the absence and presence of NBA at 303 K.
Figure 3. Potentiodynamic polarization curves for the corrosion of (a) mild steel (b) aluminium in 0.1 MHCl in the absence and presence of different concentrations of NBA.
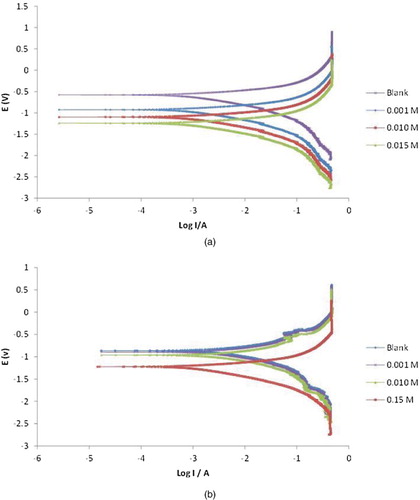
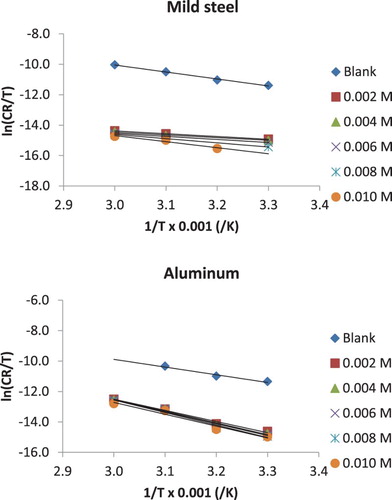
Figure 4. Arrhenius plots for the corrosion of mild steel and aluminium in 0.1 M HCl containing various concentrations of NBA.
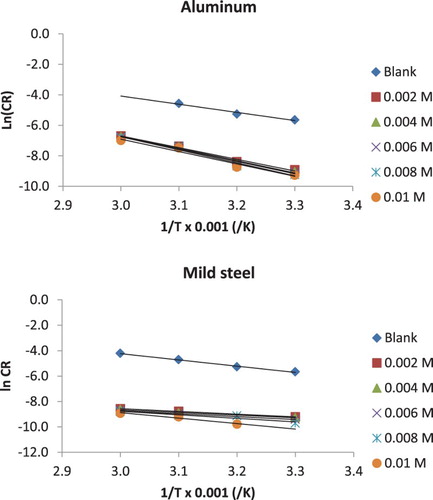
Table 3. Arrhenius and Transition state parameters for the adsorption of NBA on aluminium and mild steel surfaces.
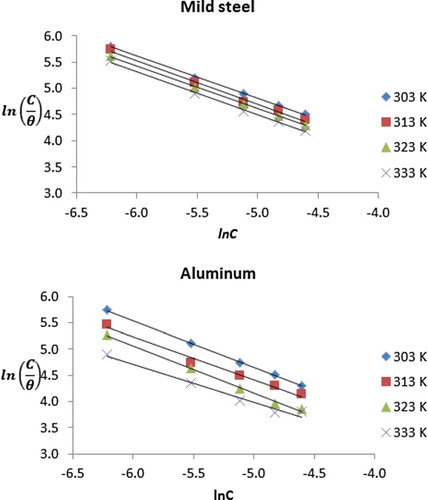
Figure 5. Transition state plots for the corrosion of mild steel and aluminium in 0.1 M HCl containing various concentrations of NBA.