Open access
2,289
Views
2
CrossRef citations to date
0
Altmetric
Research Articles
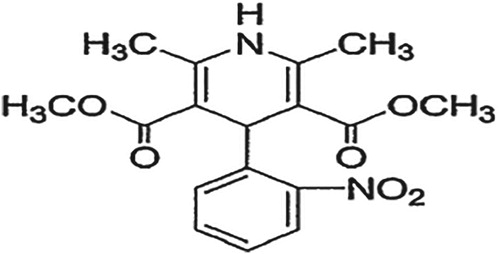
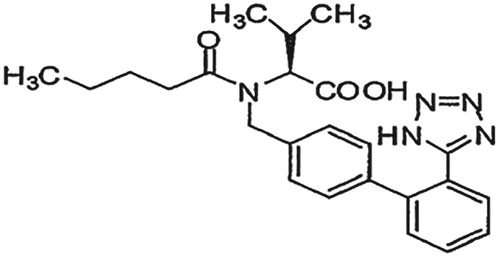
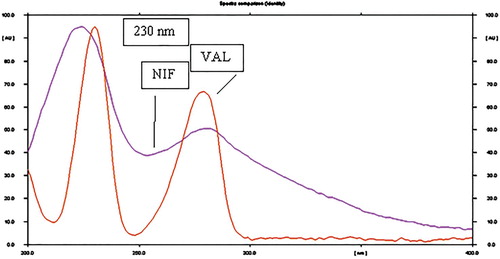
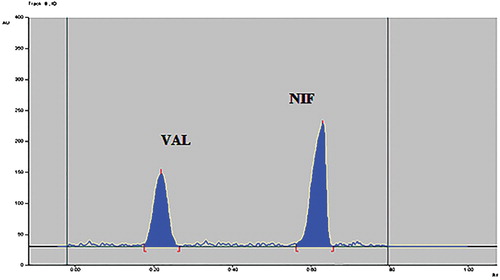
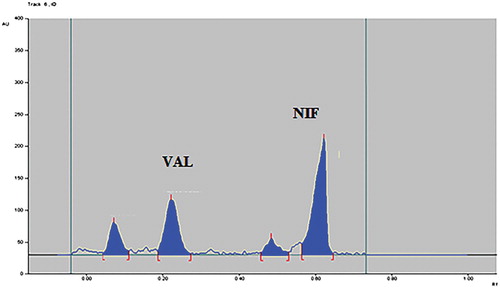
Development of stability indicating HPTLC method for estimation of antihypertensive drug combination nifedipine and valsartan
Dimal A. ShahBabaria Institute of Pharmacy, Vadodara, Gujarat, IndiaCorrespondence[email protected]
 https://orcid.org/0000-0002-6358-3739
https://orcid.org/0000-0002-6358-3739
Jaimin S. PatelIndukaka Ipcowala College of Pharmacy, Beyond GIDC, New Vallabh Vidyanagar, Gujarat, India https://orcid.org/0000-0001-6147-9711
https://orcid.org/0000-0001-6147-9711
Priyanka JadejaIndukaka Ipcowala College of Pharmacy, Beyond GIDC, New Vallabh Vidyanagar, Gujarat, India https://orcid.org/0000-0003-1893-7865
https://orcid.org/0000-0003-1893-7865
Vandana B. PatelIndukaka Ipcowala College of Pharmacy, Beyond GIDC, New Vallabh Vidyanagar, Gujarat, India https://orcid.org/0000-0003-1799-4382
https://orcid.org/0000-0003-1799-4382
Usmangani K. ChhalotiyaIndukaka Ipcowala College of Pharmacy, Beyond GIDC, New Vallabh Vidyanagar, Gujarat, India https://orcid.org/0000-0003-1183-2761
https://orcid.org/0000-0003-1183-2761
Pages 722-730
|
Received 17 Apr 2018, Accepted 16 May 2019, Published online: 10 Jun 2019
Related research
People also read lists articles that other readers of this article have read.
Recommended articles lists articles that we recommend and is powered by our AI driven recommendation engine.
Cited by lists all citing articles based on Crossref citations.
Articles with the Crossref icon will open in a new tab.